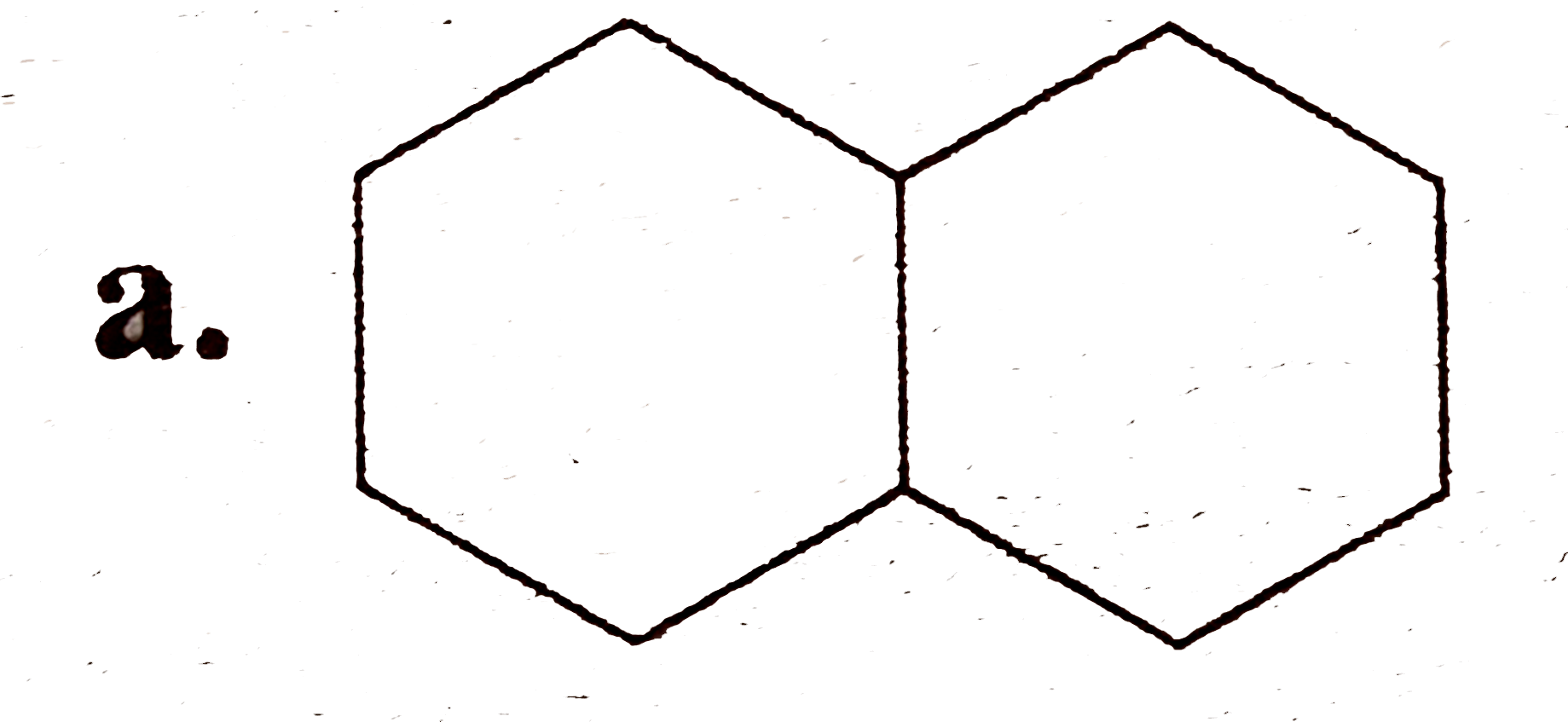
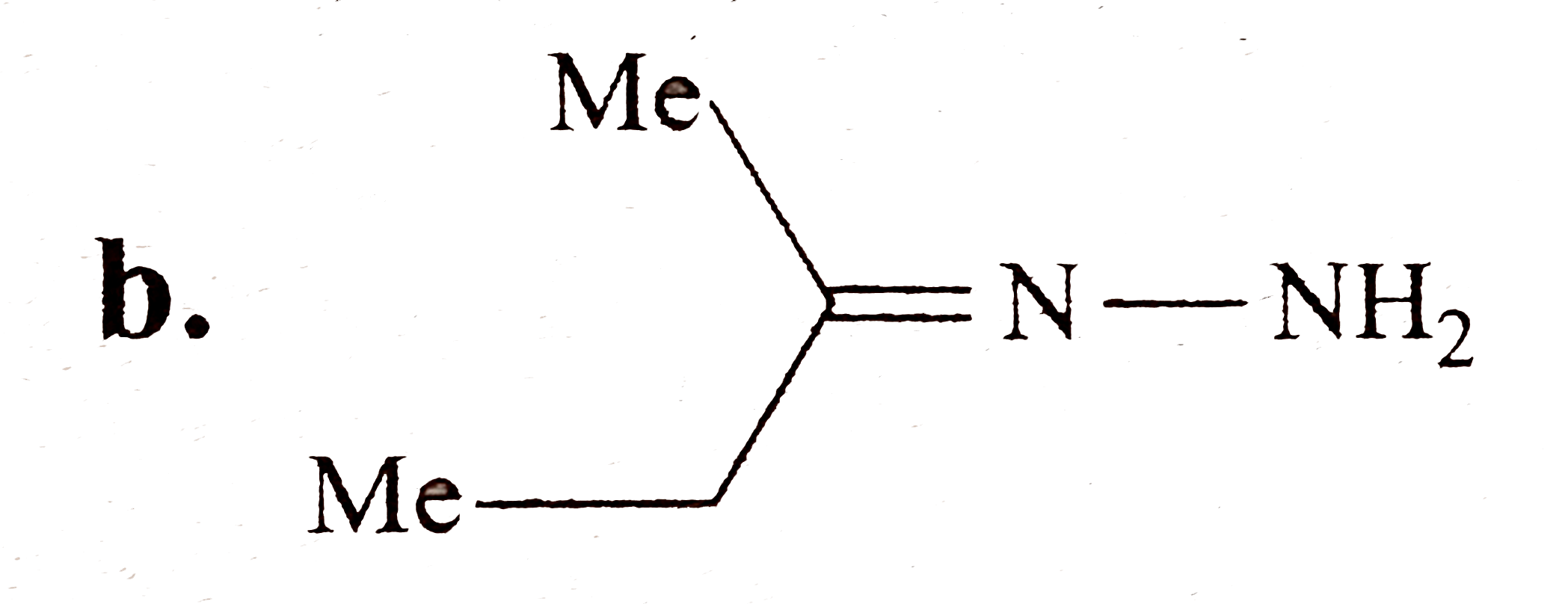
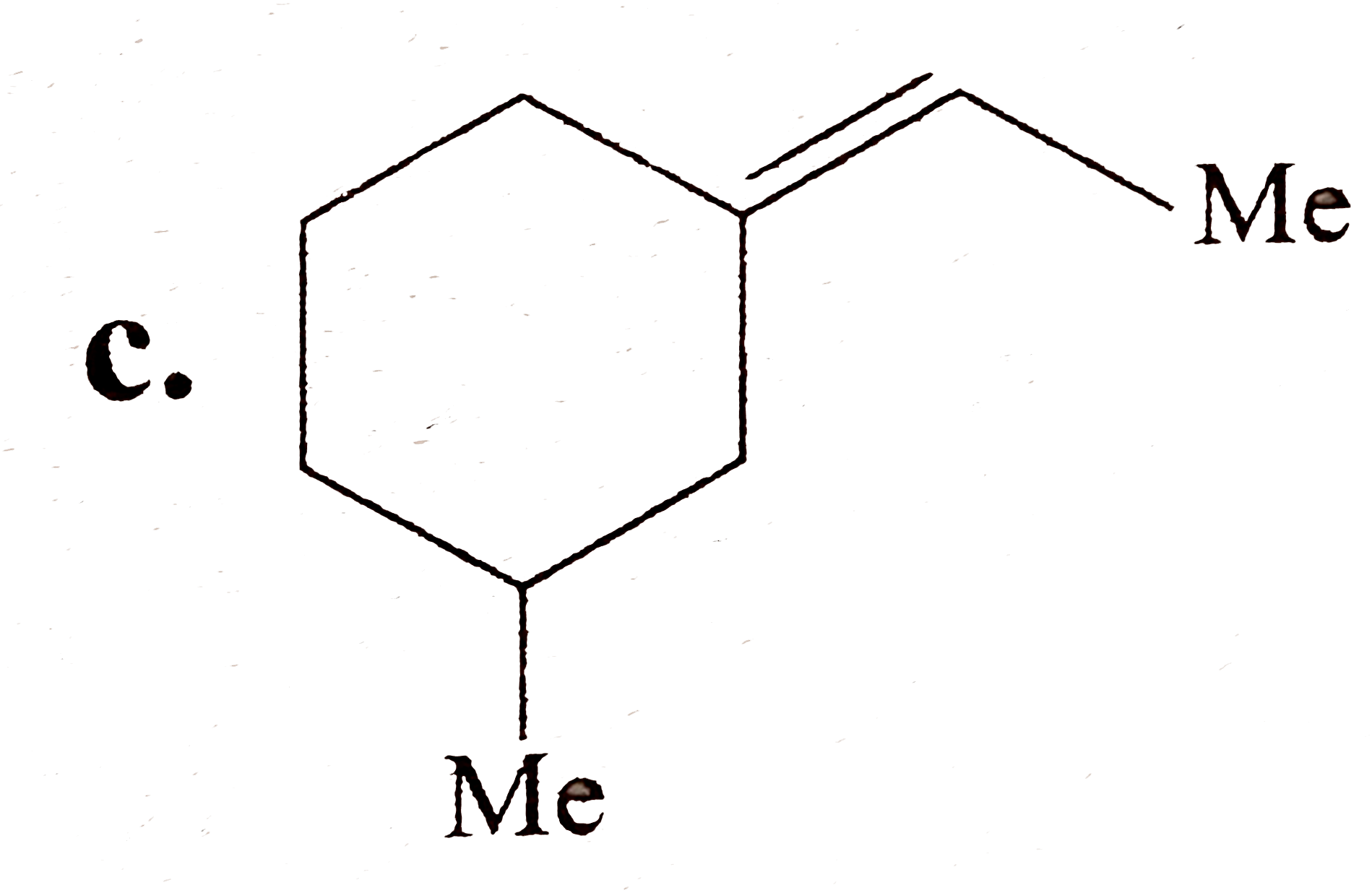
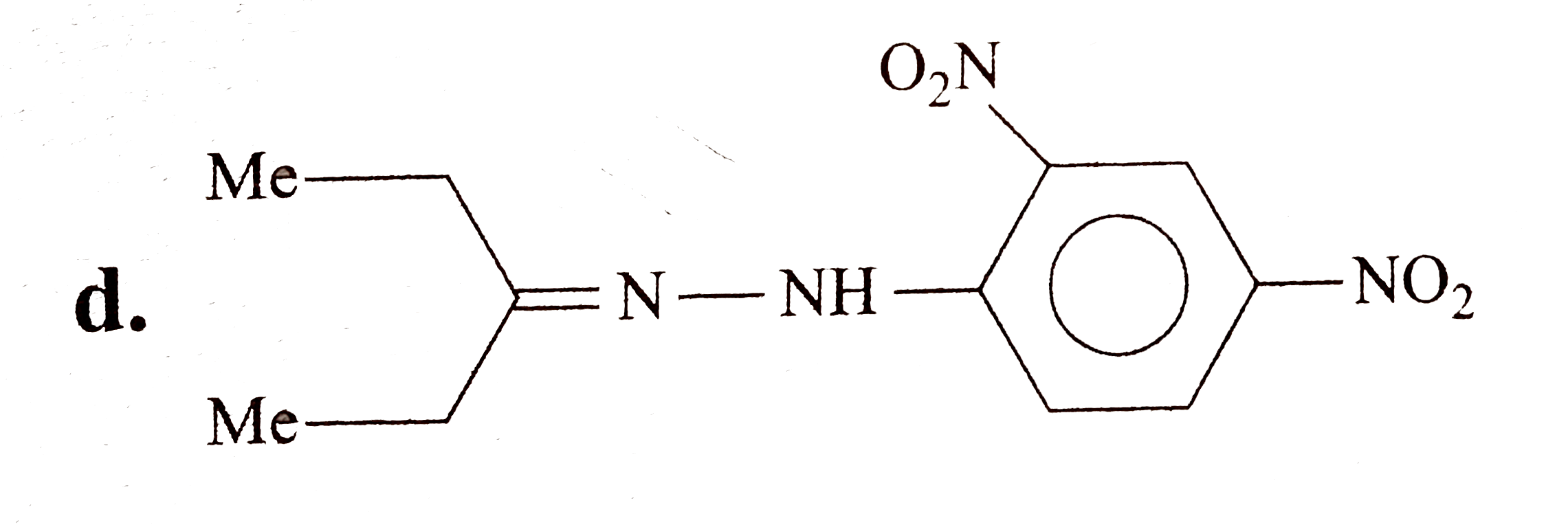A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ALKENES AND ALKADIENES
CENGAGE CHEMISTRY ENGLISH|Exercise Single Correct Answer Type|62 VideosALKENES AND ALKADIENES
CENGAGE CHEMISTRY ENGLISH|Exercise Archives(ASSERTION-REASIOING)|4 VideosALKENES AND ALKADIENES
CENGAGE CHEMISTRY ENGLISH|Exercise linked Comprehension Type|38 VideosALKANES AND CYCLOALKANES
CENGAGE CHEMISTRY ENGLISH|Exercise Archives|13 VideosALKYNES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Archives - Analytical and Desriptive Type)|4 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ALKENES AND ALKADIENES-Multiple Correct Answer Type
- Which statements is // are correct ?
Text Solution
|
- Which of the following is // are incorrectly names ?
Text Solution
|
- Which of the following show diastereomers ?
Text Solution
|
- Which of the following statement(s) is // are wrong ?
Text Solution
|
- Draw the structure of nitrobenzene
Text Solution
|
- What is Naphthalene
Text Solution
|
- Define the term Straight chain hydrocarbons
Text Solution
|
- Define the term Branched chain hydrocarbons
Text Solution
|
- Which statements is // are correct ?
Text Solution
|
- Which of the following statements are correct ?
Text Solution
|
- Which of the following statements is // are wrong ?
Text Solution
|
- Which of the following statements is/are wrong ?
Text Solution
|
- Which products are not formed ?
Text Solution
|
- What other products is // are formed in Question ?
Text Solution
|
Text Solution
|
- Which of the following dienes an dienophiles could be used to synthesi...
Text Solution
|
- Which of the following statements is //are correct.
Text Solution
|
- underset((A))underset("Propa-1,2-diene")(Coverset(3)(H)(2)=overset(2)(...
Text Solution
|
- Which of the following statements is // are correct ?
Text Solution
|
- (C) overset(H(3)O^(+)//H(2)O)(rarr)(E) (D) overset(H(3)O^(+)//H(2)O)...
Text Solution
|