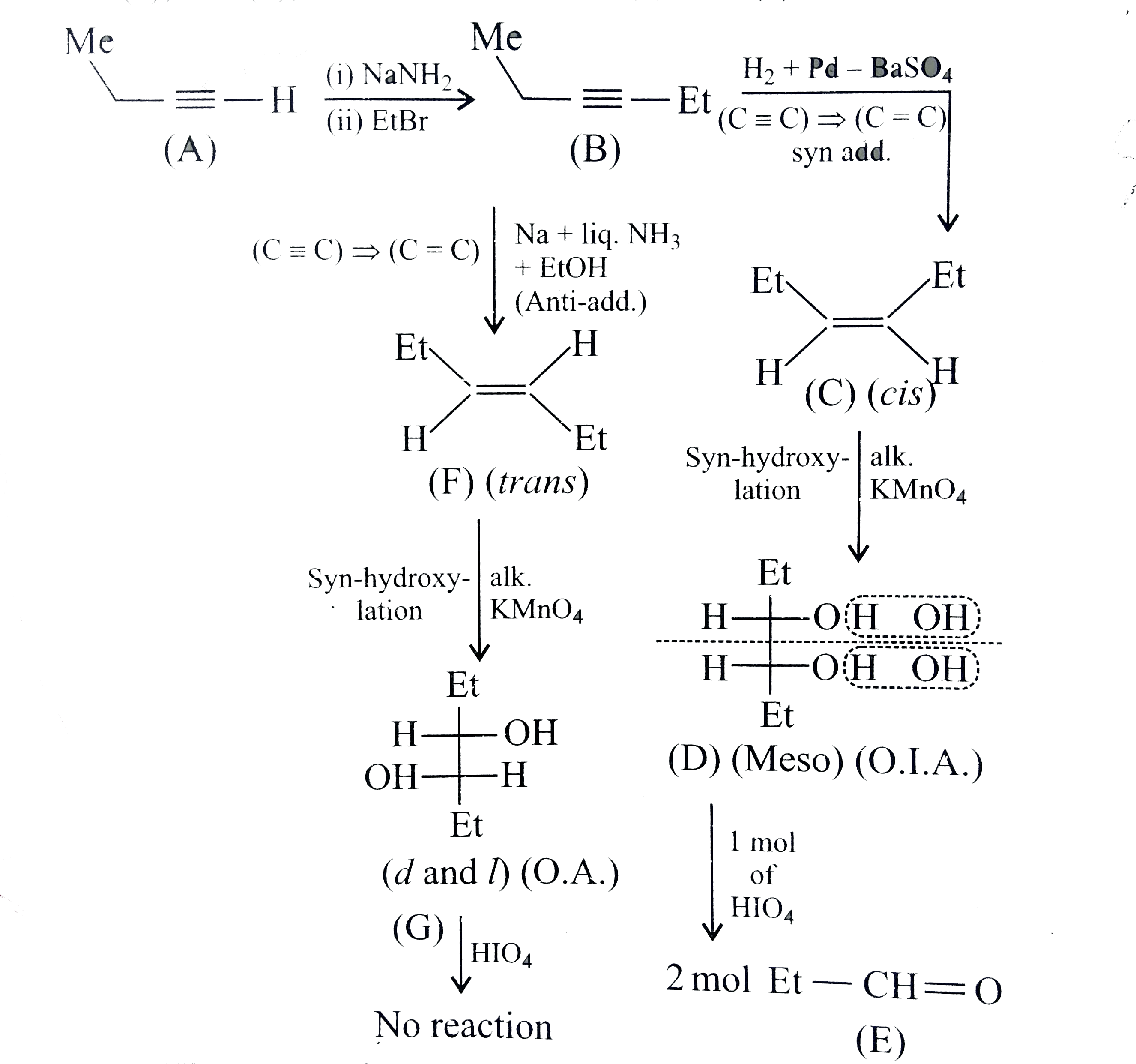
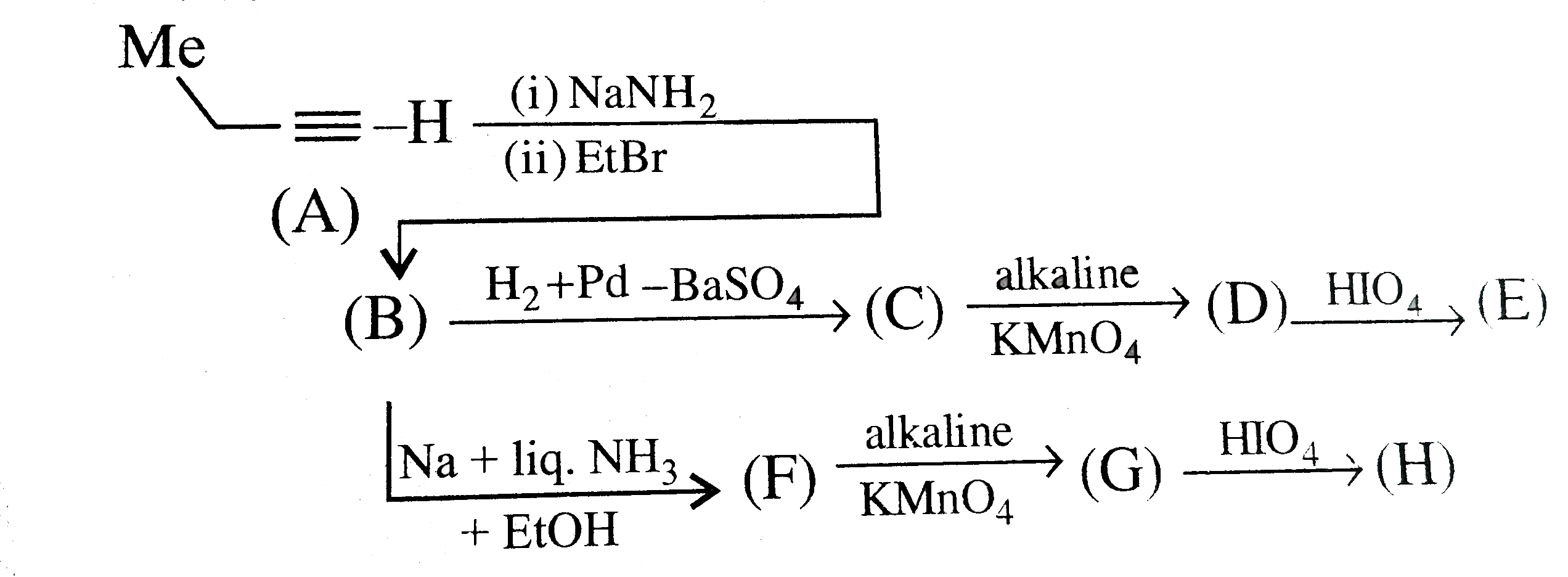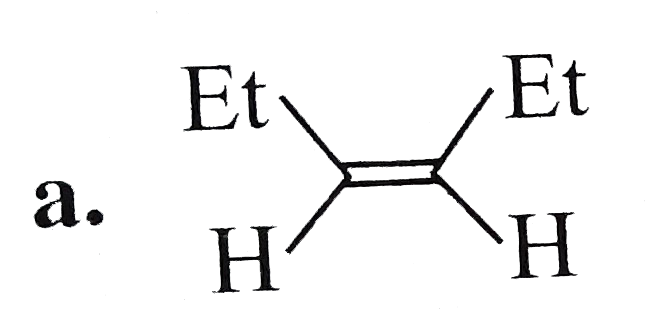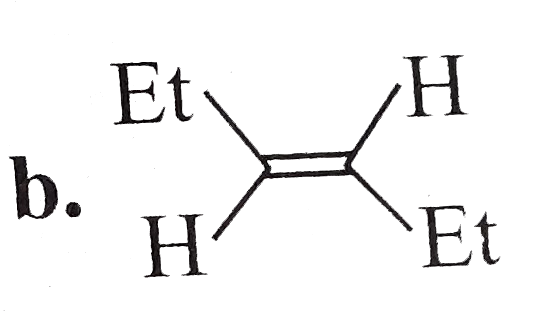A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercise (Multiple Correct)|35 VideosREDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercise (Single Correct)|90 VideosREDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise EXERCISES|1 VideosQUALITATIVE INORGANIC SALT ANALYSIS
CENGAGE CHEMISTRY ENGLISH|Exercise Viva Voce Questions And Part-C (Analysis Of Cations)|42 VideosSOLID STATE
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 1.2 (Objective)|9 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-REDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS-Exercise (Linked Comprehension)
- (A)(C(9)H(12)O)underset(Hot KMnO(4))overset([O])(rarr) PhCOOH i. (A)...
Text Solution
|
- The compound (C ) is:
Text Solution
|
- The compound (F) is:
Text Solution
|
- The compound (D) is:
Text Solution
|
- The compound (G) is:
Text Solution
|
- The compound (E) is:
Text Solution
|
- The compound (H) is:
Text Solution
|
- The compound (C ) is:
Text Solution
|
- The compound (D) is:
Text Solution
|
- The compound (E) is:
Text Solution
|
- In the formation of compoud (E) from (A), the name of the reaction is:
Text Solution
|
- The compound (F) is:
Text Solution
|
- The compound (B) is:
Text Solution
|
- The compound (C ) and (D), respectively, are:
Text Solution
|
- The compound (F) is:
Text Solution
|
- The compound (G) and (H), respectively, are:
Text Solution
|
- The compound (I) is:
Text Solution
|
- The compound (J) and (K), respectively, are:
Text Solution
|
- The compound (A) is:
Text Solution
|
- The red colour of compound (B) is due to the formation of:
Text Solution
|