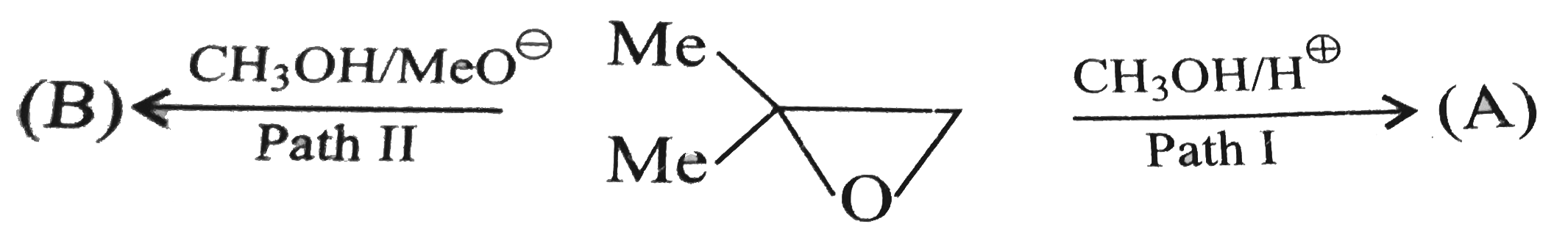
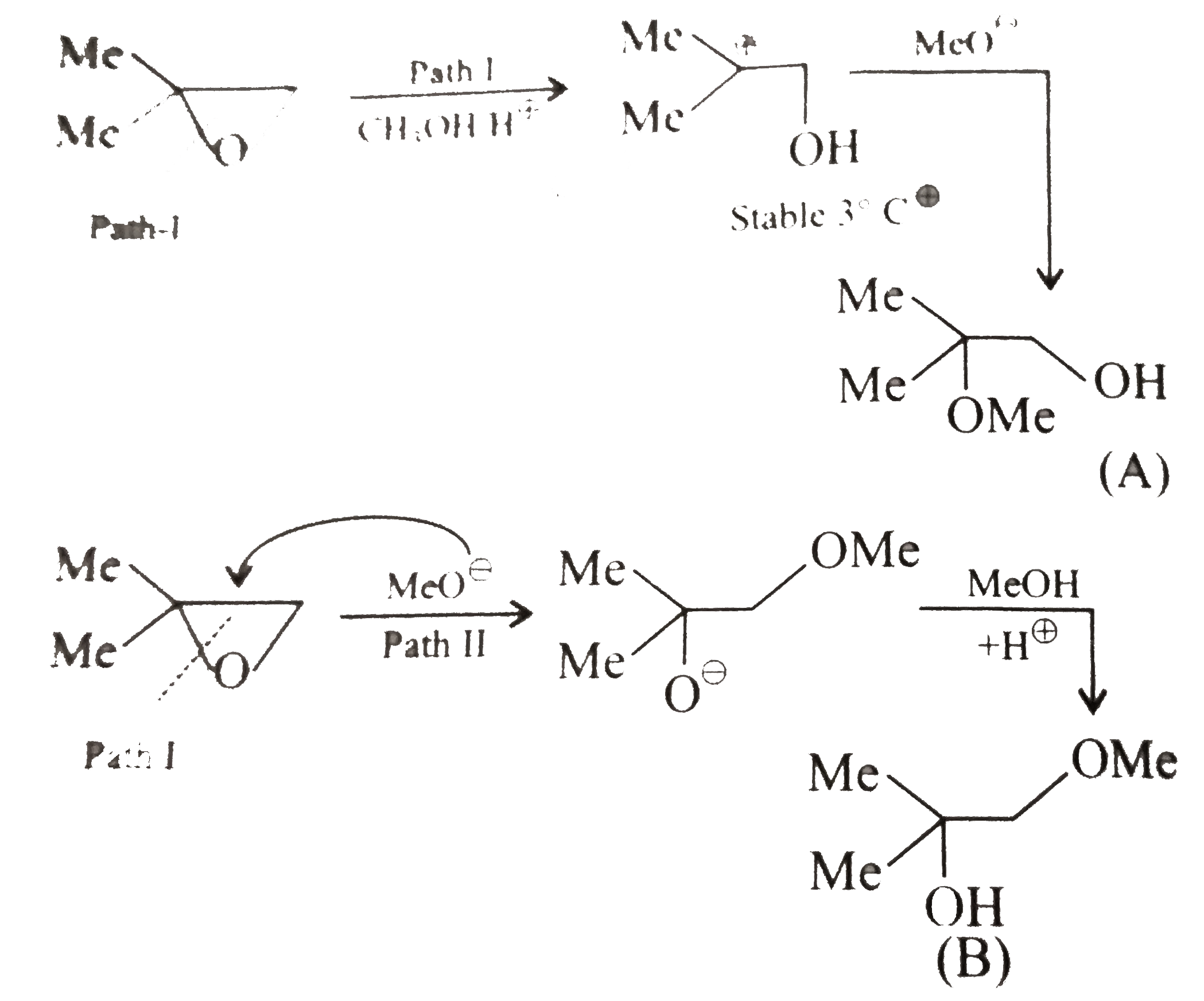
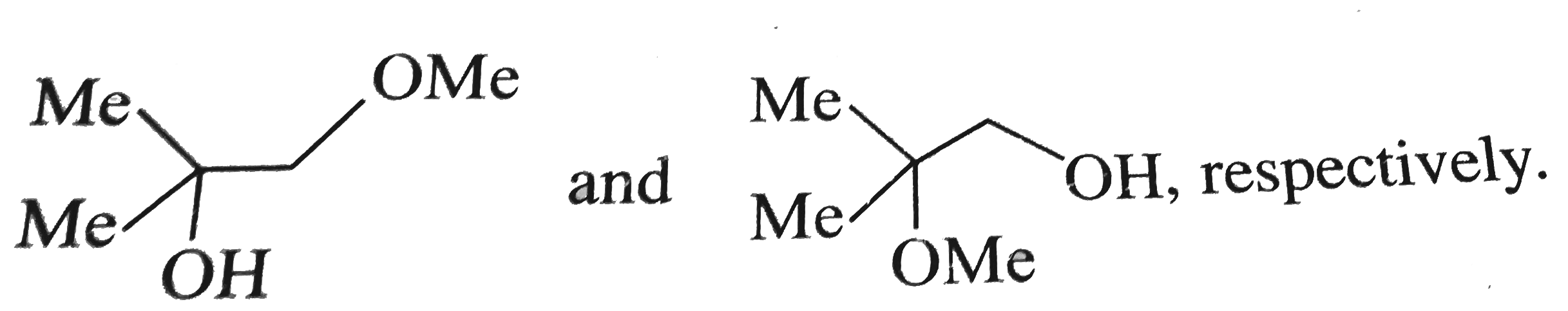A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercise Archives (Subjective)|26 VideosREDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise SUBJECTIVE TYPE|4 VideosREDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercise (Single Correct)|90 VideosQUALITATIVE INORGANIC SALT ANALYSIS
CENGAGE CHEMISTRY ENGLISH|Exercise Viva Voce Questions And Part-C (Analysis Of Cations)|42 VideosSOLID STATE
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 1.2 (Objective)|9 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-REDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS-Exercise (Assertion And Reasoning)
- Statement 1: The product (B) formed will be a racemic mixture. State...
Text Solution
|
- Statement 1: The product formed is (B). Statement 2: The reaction pr...
Text Solution
|
- The reaction of (R'-underset(O)underset(||)(C )-Cl) with (R(2)Cd) or w...
Text Solution
|
- Statement 1: The products (A) and (B) are Statement 2: Path I takes...
Text Solution
|
- Statement 1: Reduction of 3-phenyl prop-2-en-1-al with LAH gives 3-phe...
Text Solution
|
- Statement 1: Formic acid reduces 'Tollens reagent'. Statement 2: Com...
Text Solution
|
- Statement 1: tert-Butybenzene on oxidation does not give benzoic acid ...
Text Solution
|
- Statement 1: Diisopropyl ketone on reaction with isopropyl magnesium b...
Text Solution
|
- Statement 1: Schiff's regent is a dilute solution of rosaniline hydroc...
Text Solution
|
- Statement 1: Acryaldehyde (CH(2)=CH-CHO) is oxidised to acrylic acid ...
Text Solution
|