A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Multiple Correct Answers Type|15 VideosAROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Single Correct Answer Type|28 VideosAROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Linked Compreshension Type|11 VideosAPPENDIX INORGANIC VOLUME 2
CENGAGE CHEMISTRY ENGLISH|Exercise Short Answer Type|179 VideosBIOMOLECULES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (Analytical And Descriptive)|8 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES -Exercises Multiple Correct
- Which of the following reactions are both sterospecific and steroselec...
Text Solution
|
- Which of the following reactions(s) is/are both non-sterospecific but ...
Text Solution
|
- Which of the following reactions(s) is/are sterospecific but non-stero...
Text Solution
|
- Which of the following reaction(s) is/are neither sterospecific nor st...
Text Solution
|
- The first steps of SN^(1) and SN^(2) recatiosns are, respectively
Text Solution
|
- Which of the following staements are correct about ElcB reaction ?
Text Solution
|
- In which fo the following reactions is the correct major product forme...
Text Solution
|
- Which of the following syntheses could not be doen without involving b...
Text Solution
|
- Which of the following side chain reaction/s can be used to reduce th...
Text Solution
|
- In the following reactions: Which of the following staements a...
Text Solution
|
- Which are the sources of phenol?
Text Solution
|
- Which contenet (s) of middle oil separate on cooling?
Text Solution
|
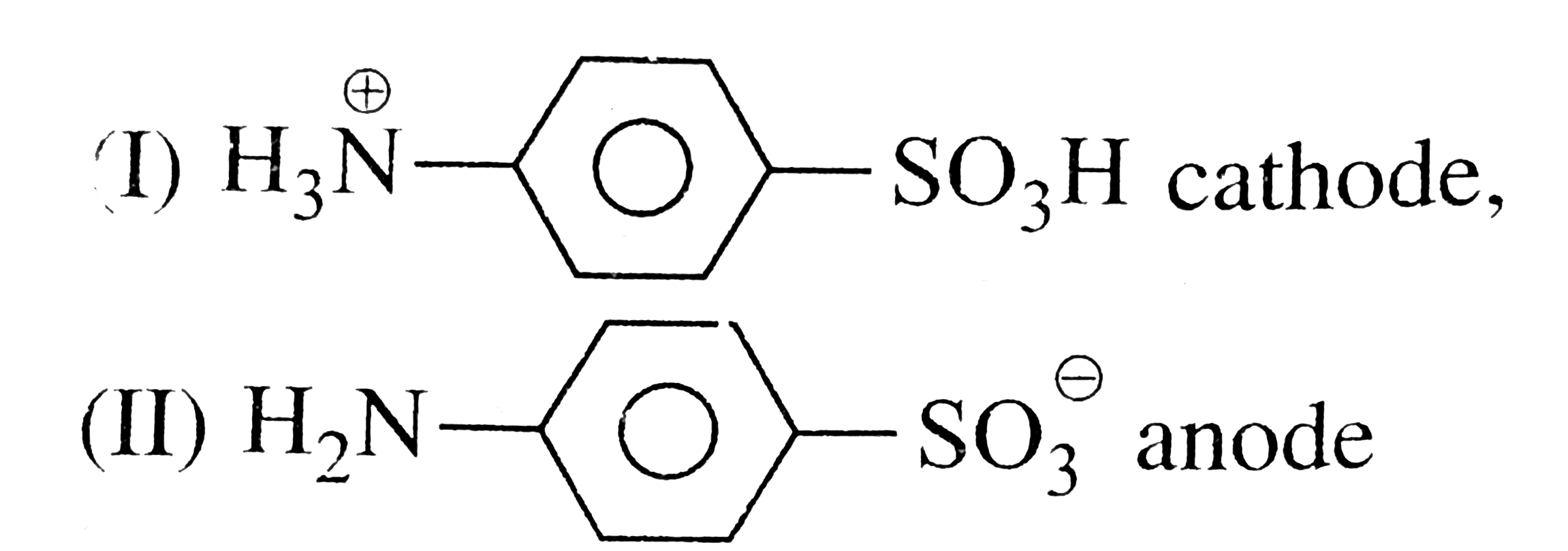
- Sulfanillic acid at pH = 2 and 12 exists as....and migrates towards......
Text Solution
|
- Consider the following reactions: I. C(2)H(5) - I + NH(3) rarr C(2) ...
Text Solution
|
- In which of the above reactions does the rate fo SN^(2) reactions dec...
Text Solution
|
- Consider the folliwng reactions: I. Me(3)C - Br underset(SN^(1))over...
Text Solution
|
- Which benzene sulphonic acid and p-nitrophenol are treated with NaHCO3...
Text Solution
|
- The decreasing order of pK(a) value of the folliwng is:
Text Solution
|
- Among the following, which is/are correct?
Text Solution
|
- Which of the following will give Hofamann alkene?
Text Solution
|