Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RC MUKHERJEE-OXIDATION NUMBER AND BALANCING OF REDOX REACTIONS -PROBLEMS
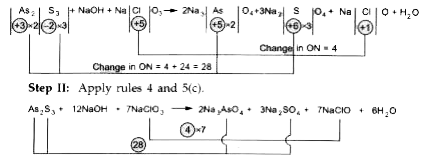
- Balance : As(2) S(3) + NaCl O(3) to Na(2) As O(4) + Na(2) SO(4) + Na...
Text Solution
|
- C(2) H(5) OH + Cr(2) O(7)^(2-) + H^(+)= Cr^(3+) + C(2) H(4) O + H(2)O
Text Solution
|
- Sn(OH)(3)^(-) + Bi(OH)(3) + OH^(-) = Sn (OH)(6)^(2-) + Bi
Text Solution
|
- IO(3)^(-) + N(2) H(4) + HCl= N(2) + I Cl(2)^(-) = N(2) I Cl(2)^(-) + H...
Text Solution
|
- NO(2) + OH^(-) = NO(3)^(-) + NO(2)^(-) + H(2)O
Text Solution
|
- Hg(2) Cl(2) + NH(3) = Hg + Hg NH(2) Cl + NH(4) Cl
Text Solution
|
- Zn + NO(3)^(-) + H^(+) = Zn^(2+) + NH(4)^(+) + H(2)O
Text Solution
|
- I(2) + NO(3)^(-) + H^(+) = Zn^(2+) + NH(4)^(+) + H(2)O
Text Solution
|
- MnO(4)^(-) + SO(2)^(2-) + H(2)O = MnO(2) + SO(4)^(2-) + OH^(-)
Text Solution
|
- H(2) O(2) + ClO(2) + OH^(-) = ClO(2)^(2-) + OH^(-)
Text Solution
|
- ClO^(-) + CrO(2)^(2-) + OH^(-) = Cl^(-) + CrO(4)^(2-) + H(2)O
Text Solution
|
- I(2) + Cl(2) + H(2) O = HIO(3) + HCl
Text Solution
|
- Cl(2) + KOH = KOCl + KCl + H(2)O
Text Solution
|
- Cl(2) + KOH = KClO(3) + KCl + H(2) O
Text Solution
|
- H(2) O(2) + I(2) = HIO(3) + H(2)O
Text Solution
|
- H(2) O(2) + KMnO(4) = MnO(2) + KOH + O(2) + H(2)O
Text Solution
|
- HNO(2) + KMnO(4) + H(2) SO(4) = HNO(3) + KMnO(4) + K(2) SO(4) + H(2)O
Text Solution
|
- NaNO(2) + NaI + H(2) SO(4) = NO + I(2) + Na(2) SO(4) + H(2)O
Text Solution
|
- N(2) H(4) + AgNO(3) + KOH = N(2) + Ag + KNO(3) + H(2) O
Text Solution
|
- N(2) H(4) + Zn + KOH + H(2) O = NH(3) + K(2) [ Zn (OH)(4)]
Text Solution
|
- Fe + N(2) H(4) + H(2) O = Fe (OH)(2) + NH(3)
Text Solution
|