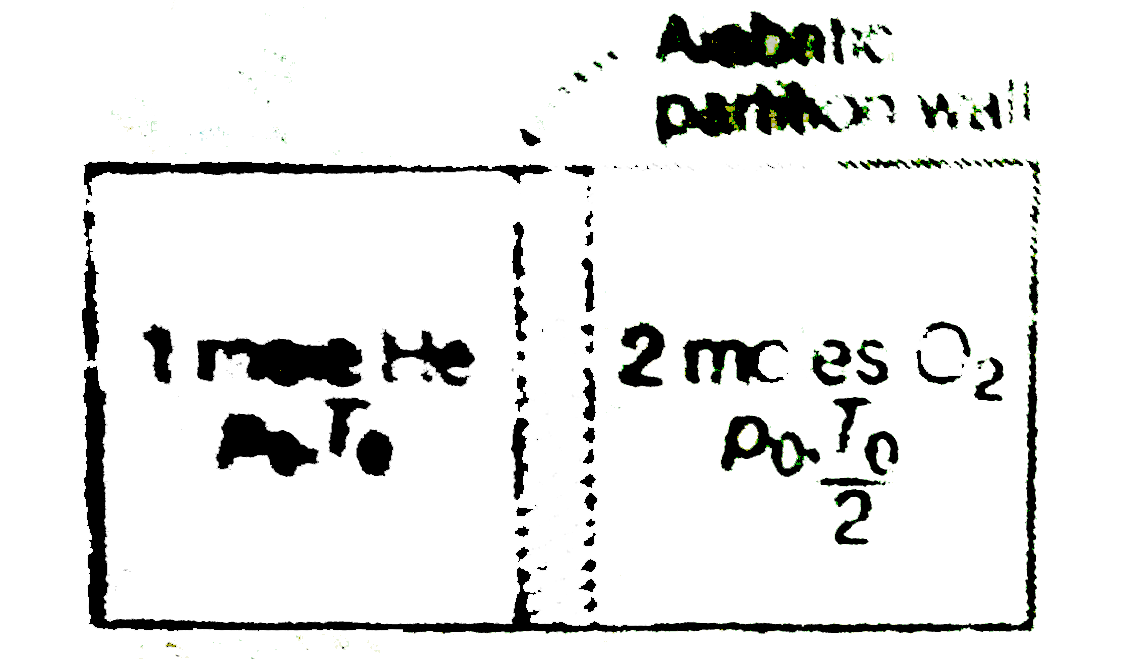
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CENGAGE PHYSICS-THERMODYNAMICS-QUESTION BANK
- The narrow tube with one of its ends sealed as shown in the figure, i...
Text Solution
|
- The figare shows two paths that may be taken by a gas from an initial ...
Text Solution
|
- Consider the adjacent figure. A piston divides a cylindrical container...
Text Solution
|
- A glass tube of length 76 cmi is in inverted position. A column of air...
Text Solution
|
- The amount of work done by a gas which is being isobarically heated fr...
Text Solution
|
- One mole of an ideal monoatomic gas is taken from state A to state B t...
Text Solution
|
- One mole of monatomic gas is taken through cyclic process shown below....
Text Solution
|
- An ideal monatounic gas of mass M is taken through a cyclic process A ...
Text Solution
|
- In a cyclic thermodynamio process, an ideal gas is first compressed at...
Text Solution
|
- Some gas (C(p) / C(v)=y=1.25) follows the cyclo A B C D A as shown in ...
Text Solution
|
- One mole of helium in a vessel gets heat from outside and starts expan...
Text Solution
|
- One mole of monatomic ideal gas undergoes the process ArarrB , as in t...
Text Solution
|
- The internal energy of monatomic ideal gas is 1.5 (MR) T. One mole of ...
Text Solution
|
- The graph shows variation of internal energy U with density rho of one...
Text Solution
|
- A cylinder of cross-section area. A has two pistons of negligible mass...
Text Solution
|
- The graph shows the P V diagram of a process carried out with a certai...
Text Solution
|
- A vessel of volume V(0) is evacuated by means of a piston air pump. On...
Text Solution
|
- There is 5 g of a certain diatomic gas in a container closed with a fr...
Text Solution
|
- A quantity of 2 mol of helium gas undergoes a thermodynamic process, i...
Text Solution
|
- 100 mol of an ideal monatomic gas undergoes the following thermodynami...
Text Solution
|