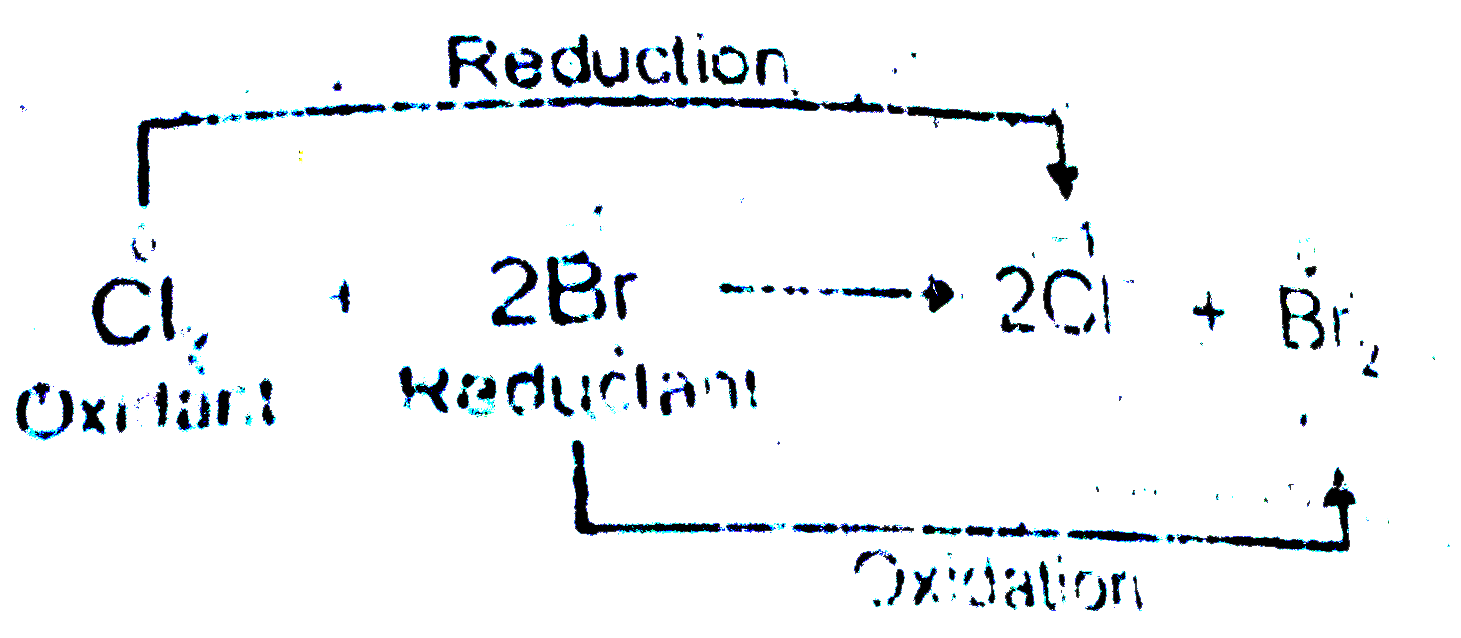Text Solution
Verified by Experts
Topper's Solved these Questions
REDOX REACTIONS
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section A) (Objective type Questions (one option is correct))|40 VideosREDOX REACTIONS
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section B) (Objective type Questions (one option is correct))|32 VideosREDOX REACTIONS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT SECTION - D|10 VideosPRINCIPLES OF QUALITATIVE ANALYSIS
AAKASH INSTITUTE ENGLISH|Exercise Assignment (SECTION H)|9 VideosSOLUTIONS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGMENT (SECTION-J) AAKASH CHALLENGERS QUESTIONS|10 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-REDOX REACTIONS-Try Yourself
- Find the oxidation number of Cl in HCl, HClO,ClO(4)^(ө), and Ca(Ocl)Cl...
Text Solution
|
- What is the oxidation number of the underlined atoms in the following ...
Text Solution
|
- Identify the oxidant and the reductant respectively in the following r...
Text Solution
|
- Is the reaction BaO(2)+H(2)SO(4)rarrBaSO(4)+H(2)O(2) a redox reaction?
Text Solution
|
- Write the disproportionation reaction of ClO^(-) to Cl^(-) and ClO(3)...
Text Solution
|
- Classify the reaction (i) 2NO(2) + 2OH^(-) to NO(2)^(-)+H(2)O (i...
Text Solution
|
- In the balanced equation MnO(4)^(-)+H^(+)+C(2)O(4)^(2-)rarr Mn^(2+)+...
Text Solution
|
- Balance the following reaction by ion electrons method ( acidic medium...
Text Solution
|
- Balance the equation in acidic medium H(2)S + Fe^(3+) to Fe^(3+) + ...
Text Solution
|
- Balance the equation by lon-electron method Al + NO(3)^(-) to Al(OH...
Text Solution
|
- Why blue colour of CuSO(4) solution gets discharged when zinc rod is d...
Text Solution
|
- Can a solution of 1 M ZnSO(4) be stored in a vessel made of copper ? G...
Text Solution
|
- Write the half reaction for the reaction 2Fe^(+3) + 2I^(-) to 2Fe^(...
Text Solution
|
- identify oxidation and reduction process for the reaction CH(2) =CH...
Text Solution
|
- Determine the oxidation number of Cl in HCl, HOCl, HClO(4) " and " ClO...
Text Solution
|
- What is the oxidation number of the underlined atoms in the following ...
Text Solution
|
- Identify the oxidant and the reductant respectively in the following r...
Text Solution
|
- Is this a redox reaction ? BaO(2) + H(2)SO(4) to BaSO(4) + H(2)O(2...
Text Solution
|
- Write the disproportionation reaction of ClO^(-) to Cl^(-) and ClO(3)...
Text Solution
|
- Classify the reaction (i) 2NO(2) + 2OH^(-) to NO(2)^(-)+H(2)O (i...
Text Solution
|