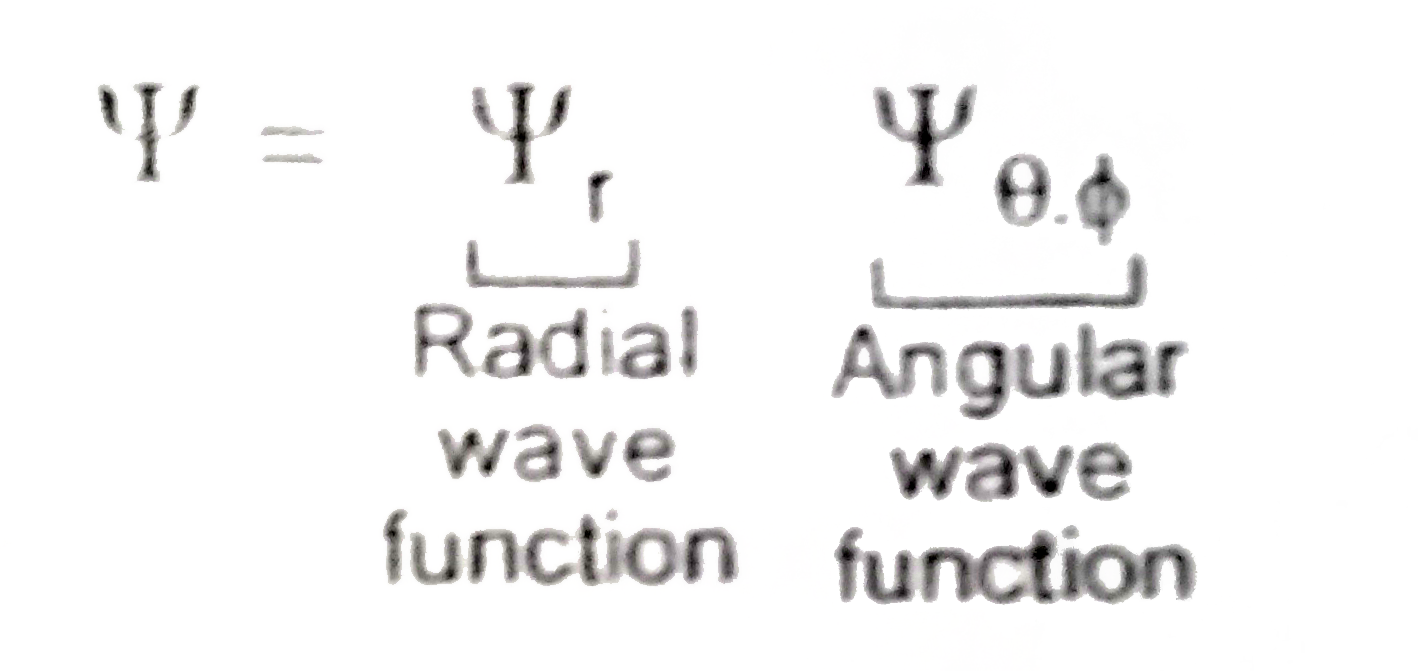A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STRUCTURE OF ATOM
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT ( SECTION -E) Assertion - Reason Type Questions|10 VideosSTRUCTURE OF ATOM
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT ( SECTION -F) Matrix - Match Type Questions|4 VideosSTRUCTURE OF ATOM
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT ( SECTION -C) Objective Type Questions (More than one options are correct)|15 VideosSTRUCTURE OF ATOM
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (SECTION -D) Assertion-Reason Type Questions|15 VideosSURFACE CHEMISTRY
AAKASH INSTITUTE ENGLISH|Exercise Assignment Section - C (Assertion - Reason type questions)|10 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-STRUCTURE OF ATOM -ASSIGNMENT ( SECTION -D) Linked Comprehension Type Questions)
- The energy of n^(th) orbit is given by E(n) = ( -Rhc)/(n^(2)) Whe...
Text Solution
|
- The energy of n^(th) orbit is given by E(n) = ( -Rhc)/(n^(2)) Whe...
Text Solution
|
- The energy of n^(th) orbit is given by E(n) = ( -Rhc)/(n^(2)) Whe...
Text Solution
|
- In photoelectric effect light of certain frequency ( gt threshold fre...
Text Solution
|
- In photoelectric effect light of certain frequency ( gt threshold fre...
Text Solution
|
- In photoelectric effect light of certain frequency ( gt threshold fre...
Text Solution
|
- Orbitals are the pictorial representationofPsi or Psi ^(2) Psi^(2...
Text Solution
|
- Orbitals are the pictorial representationofPsi or Psi ^(2) Psi^(2...
Text Solution
|
- Orbitals are the pictorial representationofPsi or Psi ^(2) Psi^(2...
Text Solution
|