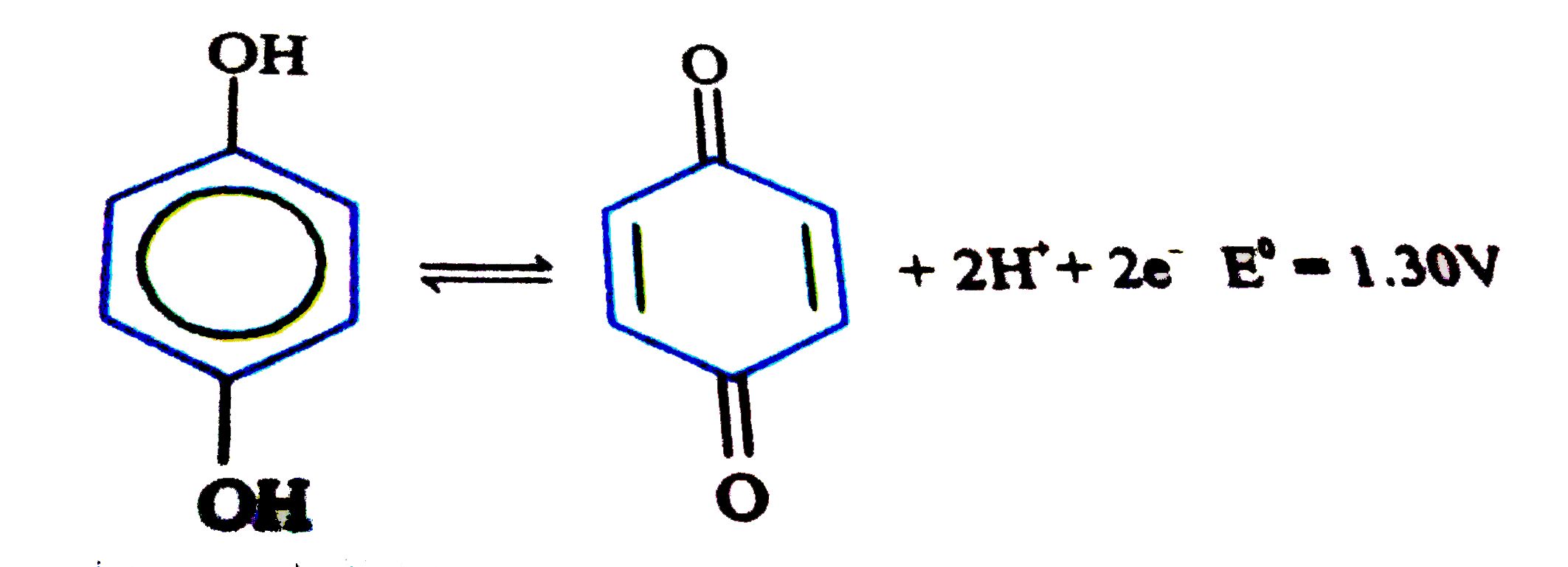A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
AAKASH INSTITUTE ENGLISH|Exercise Assignment (SECTION C) Objective Type Questions (More than one option are correct)|16 VideosELECTROCHEMISTRY
AAKASH INSTITUTE ENGLISH|Exercise Assignment (SECTION D) Linked Comprehension Type Questions|6 VideosELECTROCHEMISTRY
AAKASH INSTITUTE ENGLISH|Exercise Assignment (SECTION A) Objective Type Questions (One option is correct)|43 VideosCOORDINATION COMPOUNDS
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section-J Aakash Challenger Questions )|10 VideosENVIRONMENTAL CHEMISTRY
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (SECTION-D) (Assertion - Reason Type Questions)|4 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-ELECTROCHEMISTRY -Assignment (SECTION B) Objective Type Questions (One option is correct)
- The equilibrium constant for the reaction Sr(s)+Mg^(+2)(aq)rarr Sr^(+2...
Text Solution
|
- 0.5 faraday of electricity was required to deposit all the copper in 5...
Text Solution
|
- Cu^(+) ion is not stable in aqueous solution because because of dispro...
Text Solution
|
- 25 gm of a metal is deposited on cathode during the electrolysis of me...
Text Solution
|
- A well stirred solution of 0.1 M CuSO(4) is electrolysed at 25^(@)C us...
Text Solution
|
- 50 ML of a buffer of 1 M NH(3) and 1 M NH(4)^(+) are placed in two vol...
Text Solution
|
- The specific conductance of saturated solution os silver chloride is k...
Text Solution
|
- The limiting equivalent conductivity of NaCl, KCl and KBr are 126.5,1...
Text Solution
|
- The equivalent conductances of CH(3)COONa, HCI "and" NaCI at infinit e...
Text Solution
|
- E^(@)(Na^(+)//Na)=-2.71V E^(@)(Mg^(+2)//Mg)=-2.37V E^(@)(Fe^(+2)//Fe...
Text Solution
|
- Which of following type of plot would you expect from the titratin of ...
Text Solution
|
- The standard reduction potential of Cu^(2+)//Cu and Cu^(2+)//Cu^(+) ar...
Text Solution
|
- What is DeltaG^(@) for the following reaction Cu^(+2)(aq)+2Ag(s)rarr...
Text Solution
|
- For the half cell At pH = 3 electrode potential is
Text Solution
|
- Rate of corrosion I maximum when
Text Solution
|
- H(2)(1 "atm")|2.26 M HCOOH||0.222MCH(3)COOH|(1 "atm")H(2)K(a)(HCOOH)=...
Text Solution
|
- Given that NiO(2)+4H^(+)+2e^(-)rarrNi^(2+)+2H(2)O,E^(@)=1.678V NiO...
Text Solution
|
- Zn amalgam is prepared by elctrtolysis of aqueous ZnCI(2) using 9 gram...
Text Solution
|
- Emf of cell given Ag(s),Ag(s)|KCI(aq)Hg(2)CI(2)(s)|Hg(s) s 0.05 V ...
Text Solution
|
- Dissociation constant for Ag(NH(3))(2)^(+) into Ag^(+) and NH(3) is 6 ...
Text Solution
|