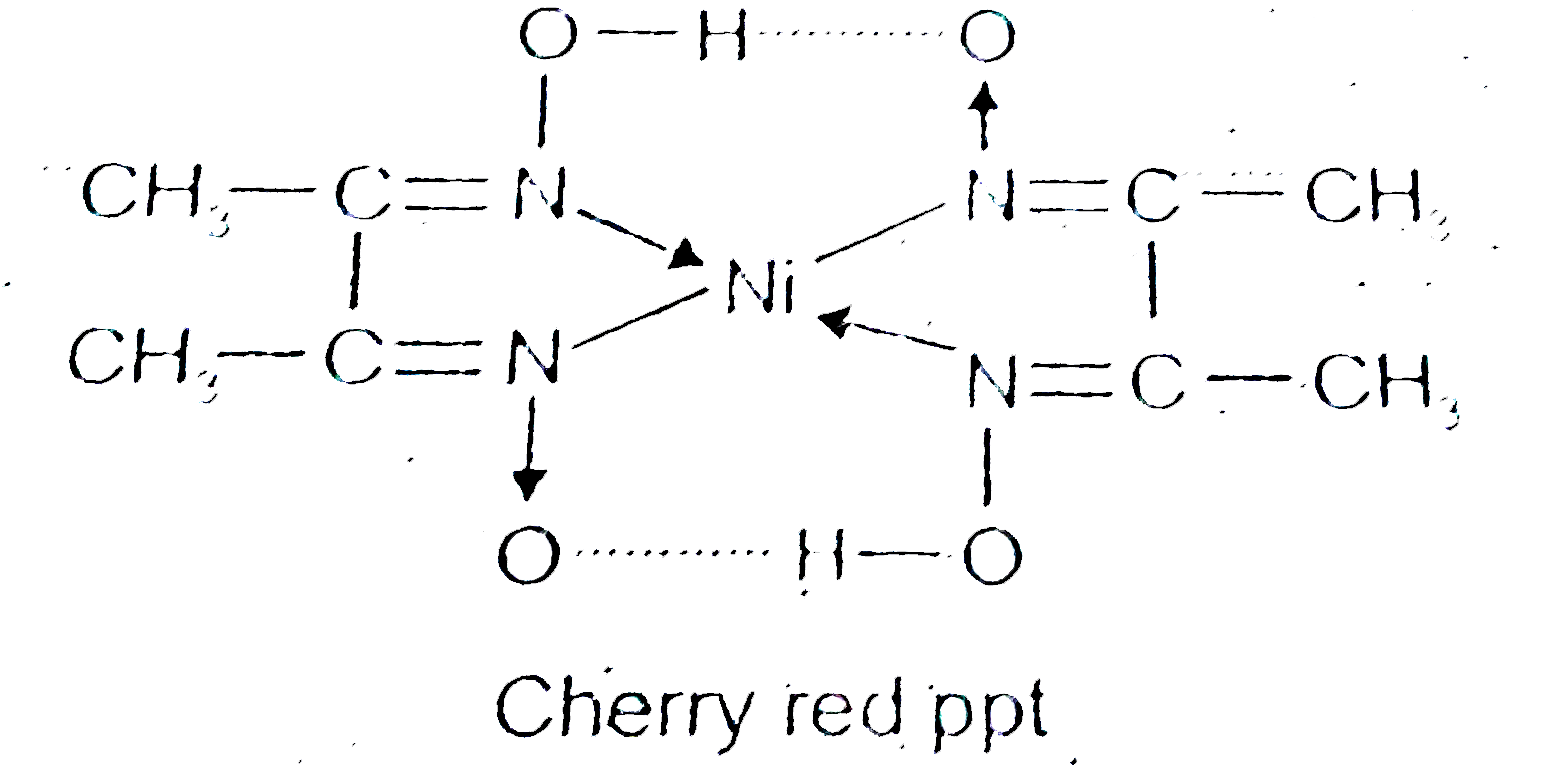Text Solution
Verified by Experts
Topper's Solved these Questions
COORDINATION COMPOUNDS
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section-J Aakash Challenger Questions )|10 VideosCOORDINATION COMPOUNDS
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section-H Multiple True-False Type Questions )|5 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section - J Aakash Challengers Questions)|6 VideosELECTROCHEMISTRY
AAKASH INSTITUTE ENGLISH|Exercise Assignment (SECTION - J) (Aakash Challengers Questions)|10 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-COORDINATION COMPOUNDS -Assignment (Section-I Subjective Type Questions )
- The cell reaction of a cell is as follows: Mg(s)+Cu(aq)^(2+) →Cu(s)+Mg...
Text Solution
|
- Calculate the emf of the cell Cr∣Cr^(3+)(0.1 M)∣∣Fe^(2+)(0.01M)∣Fe (G...
Text Solution
|
- For the following cell, Calculate the emf: Al/Al^(3+) (0.01M)//Fe^(2+)...
Text Solution
|
- Account for the following (i) Co(II) is stable in aqueous solution ...
Text Solution
|
- FeSO4 solution mixed with (NH4)2SO4 solution is 1:1 molar ratio gives ...
Text Solution
|
- (a) Square planar complexes with coordination number four exhibit geo...
Text Solution
|
- The equilibrium constant for the following reaction at 25^(@)C is 2.9×...
Text Solution
|
- Classify each of the following complexes as either high or low spin ....
Text Solution
|
- Write the IUPAC nomenclature of the given complex along with its hybri...
Text Solution
|
- NiCI(2) in the presence of dimethy1 glyoxime (DMG) gives a complex whi...
Text Solution
|