'Stability of carbocations depends upon the electron releasing inductive effect of groups adjacent to positively charged atom involvement of neighbouring groups in hyperconjugation and resonace''.
The structure of triphenylmethyl cation is given below. This is very stable and some of its salts can be stored for months. Explain the cause of high stability of this cation
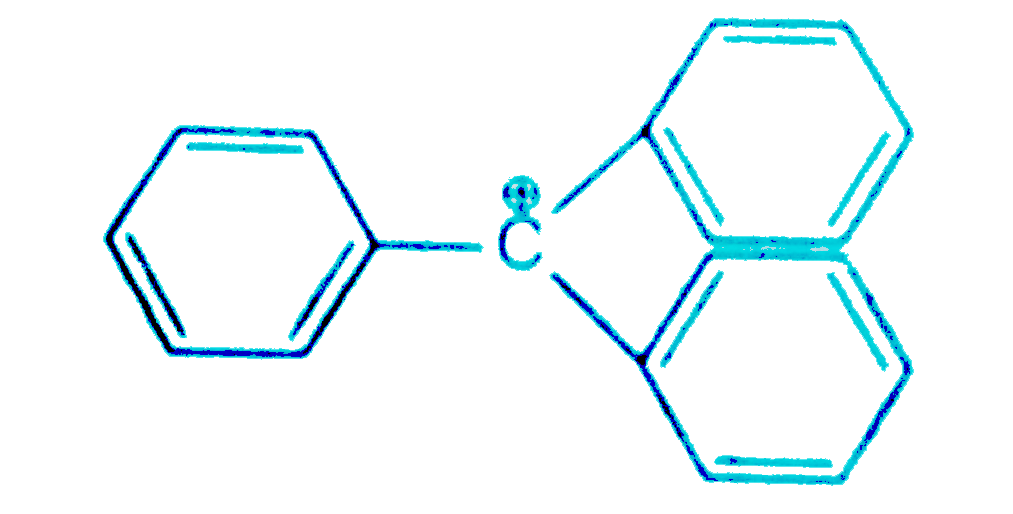
'Stability of carbocations depends upon the electron releasing inductive effect of groups adjacent to positively charged atom involvement of neighbouring groups in hyperconjugation and resonace''.
The structure of triphenylmethyl cation is given below. This is very stable and some of its salts can be stored for months. Explain the cause of high stability of this cation

The structure of triphenylmethyl cation is given below. This is very stable and some of its salts can be stored for months. Explain the cause of high stability of this cation

Text Solution
Verified by Experts
Triphenyl methyl cation is very stable, due to resonance.
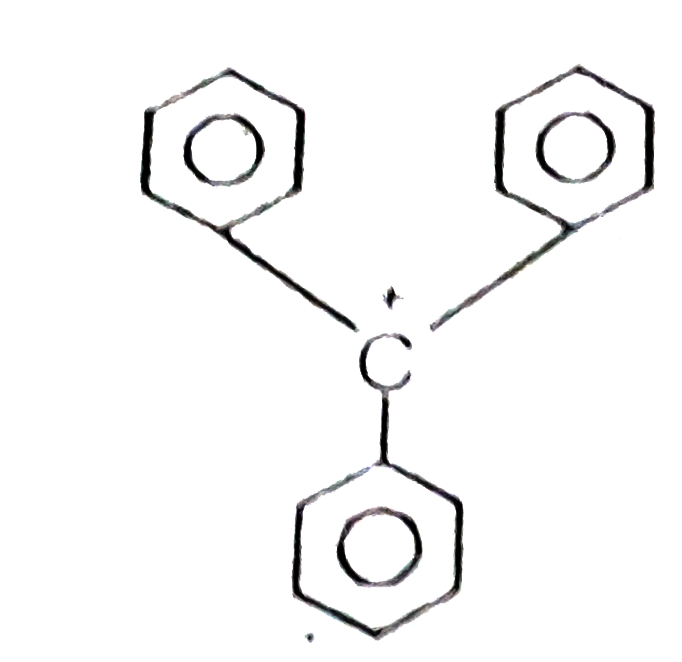 In triphenyl methyl carbocation positive charge can be deiocalized in all the 3 ring system. Causing the lowering of energy and making the carbocation very stable
In triphenyl methyl carbocation positive charge can be deiocalized in all the 3 ring system. Causing the lowering of energy and making the carbocation very stable
 In triphenyl methyl carbocation positive charge can be deiocalized in all the 3 ring system. Causing the lowering of energy and making the carbocation very stable
In triphenyl methyl carbocation positive charge can be deiocalized in all the 3 ring system. Causing the lowering of energy and making the carbocation very stableTopper's Solved these Questions
ORGANIC CHEMISTRY: SOME BASIC PRINCIPLE AND TECHNIQUES
AAKASH INSTITUTE ENGLISH|Exercise SECTION-J AAKASH CHALLENGERS QUESTIONS|10 VideosORGANIC CHEMISTRY: SOME BASIC PRINCIPLE AND TECHNIQUES
AAKASH INSTITUTE ENGLISH|Exercise TRY YOURSELF|28 VideosORGANIC CHEMISTRY: SOME BASIC PRINCIPLE AND TECHNIQUES
AAKASH INSTITUTE ENGLISH|Exercise SECTION-H MULTIPLE TRUE-FALSE TYPE QUESTION|2 VideosORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES
AAKASH INSTITUTE ENGLISH|Exercise Assignment(Section-D)(Assertion - Reason Type Questions)|15 VideosPOLYMERS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (SECTION-D)|13 Videos
Similar Questions
Explore conceptually related problems
'Stability of carbocations depends upon the electron releasing inductive effect of groups adjacent to positively charged atom involvement of neighbouring groups in hyperconjugation and resonace''. Which of the following ions is more stable ? Use resonance of explain your answer.
'Stability of carbocations depends upon the electron releasing inductive effect of groups adjacent to positively charged atom involvement of neighbouring groups in hyperconjugation and resonace''. Write structure of various carbocations that can be obtained from 2-methylbutane. Arrange these carbocations in order of increasing stability
'Stability of carbocations depends upon the electron releasing inductive effect of groups adjacent to positively charged atom involvement of neighbouring groups in hyperconjugation and resonace''. Draw the possible resonance structures for CH_(3) - underset(..)overset(..)(O) - overset(+)(C) H_(2) and predict which of the structures is more stable. Give reason for your answer.
Reaction intermdiates are short lived species and are highly reactive. They are formed by heterolytic and homolytic bond fission. There are various types of reaction intermediates in which the most important are carbocation , carbanion and free radical. Carbocation is an organic species in which carbon have positive charge and six electrons in its outermost shell. The stability of carbocation can be increased by positive inductive effect, hyperconjugation and delocalisation. If alpha -atom with respect to carbocation has one or more lone pair of electron then lone pair of electron strongly stabilises the carbocation due to octet completion. Species in which carbon have negative charge is called carbanion. Carbanion carries three bond pairs and one lone pair. The stability of carbanion can be increased by negative inductive effect, negative mesomeric effect and delocalisation. Free radical is a species which have seven electrons in its outermost shell. The stability of free radical can be increased by hyperconjugation and delocalisation. The stability order of following carbocations is
Reaction intermdiates are short lived species and are highly reactive. They are formed by heterolytic and homolytic bond fission. There are various types of reaction intermediates in which the most important are carbocation , carbanion and free radical. Carbocation is an organic species in which carbon have positive charge and six electrons in its outermost shell. The stability of carbocation can be increased by positive inductive effect, hyperconjugation and delocalisation. If alpha -atom with respect to carbocation has one or more lone pair of electron then lone pair of electron strongly stabilises the carbocation due to octet completion. Species in which carbon have negative charge is called carbanion. Carbanion carries three bond pairs and one lone pair. The stability of carbanion can be increased by negative inductive effect, negative mesomeric effect and delocalisation. Free radical is a species which have seven electrons in its outermost shell. The stability of free radical can be increased by hyperconjugation and delocalisation. The stability order of following free radicals is: C_(6)H_(5)underset(I)CH_(2)overset(*)CH_(2)" "CH_(3)underset(II)CH_(2)overset(*)CH_(2)" "underset(" "III)(C_(6)H_(5))overset(*)CH_(2)" "underset(IV)(CH_(3)^(*)
Reaction intermdiates are short lived species and are highly reactive. They are formed by heterolytic and homolytic bond fission. There are various types of reaction intermediates in which the most important are carbocation , carbanion and free radical. Carbocation is an organic species in which carbon have positive charge and six electrons in its outermost shell. The stability of carbocation can be increased by positive inductive effect, hyperconjugation and delocalisation. If alpha -atom with respect to carbocation has one or more lone pair of electron then lone pair of electron strongly stabilises the carbocation due to octet completion. Species in which carbon have negative charge is called carbanion. Carbanion carries three bond pairs and one lone pair. The stability of carbanion can be increased by negative inductive effect, negative mesomeric effect and delocalisation. Free radical is a species which have seven electrons in its outermost shell. The stability of free radical can be increased by hyperconjugation and delocalisation. Which of the following is the most stable carbanion intermediate ?
The elements of group 1 describe, more clearly than any other group of elements, the effects of increasing the size of atoms or ions on the physical and chemical properties.The chemical and physical properties the elements are closely related to their electronic structures and sizes.These metals are highly electropositive and thus form very strong bases, and have quite stable oxo-salts, In the manufacturing of sodium hydroxide chlorine and sodium carbonate, the sodium chloride is used as starting material. Which is not correctly matched ? (1)Basic strength of oxides , Cs_2OltRb_2OltK_2OltNa_2OltLi_2O (2)Stability of peroxides , Na_2O_2 lt K_2O_2 lt Rb_2O_2 lt Cs_2O_2 (3)Stability of bicarbonates , LiHCO_3 lt NaHCO_3 lt KHCO_3 lt RbHCO_3lt CsHCO_3 (4)Melting point , NaF < NaCl < NaBr < Nal
The existence of negatively charged particle in an atom was shown by J.J. Thomson as a result of the studies of the passage of electricity through gases at extremely low pressure known as discharge tube experiments. When a high voltage of the order of 10,000 volts or more was impressed across the electrodes, some sort of invisible rays moved from the negative electrode to the positive electrode these rays are called as cathode rays. Cathode rays travel in straight path in absence of electrical and magnetic field . Cathode rays consist of material part and charged particles? Cathode rays produce X-rays and light is emitted when they strike on ZnS screen. Cathode rays penetrate through thin sheets of aluminium and other metals . They affect the photogenic plate and passes heating effect when they strike on metal foil. The raito of charge to mass i.e charge/mass is same for all the cathode rays irrespective of the gas used in the tube. The existence of positively charged particle in an atom was shown be E. Goldstein. He repeated the same discharge tube experiments by using a perforated cathode. It was observed that when a high potential difference was applied between the electrodes, not only cathode rays were produced but also a new type of rays were produced simultaneoulsy from anode moving towards cathode and passes through the holes or canal of the cathode. These termed as canal rays or anode rays. These rays travel in straight lines and consists of positively charged particles. These rays have kinetic energy and produces heating effect also. The e/m ratio of these rays is smaller than that of electrons. Unlike cathode rays, their e/m value is dependent upon the nature of the gas taken in the tube. These rays produced flashes of light on ZnS screen and can pass throughs thin metal foils. They can produce physical and chemical changes and are capable to produce ionisation in gases. Which is not true with respect to cathode rays?
The existence of negatively charged particle in an atom was shown by J.J. Thomson as a result of the studies of the passage of electricity through gases at extremely low pressure known as discharge tube experiments. When a high voltage of the order of 10,000 volts or more was impressed across the electrodes, some sort of invisible rays moved from the negative electrode to the positive electrode these rays are called as cathode rays. Cathode rays travel in straight path in absence of electrical and magnetic field . Cathode rays consist of material part and charged particles? Cathode rays produce X-rays and light is emitted when they strike on ZnS screen. Cathode rays penetrate through thin sheets of aluminium and other metals . They affect the photogenic plate and passes heating effect when they strike on metal foil. The raito of charge to mass i.e charge/mass is same for all the cathode rays irrespective of the gas used in the tube. The existence of positively charged particle in an atom was shown be E. Goldstein. He repeated the same discharge tube experiments by using a perforated cathode. It was observed that when a high potential difference was applied between the electrodes, not only cathode rays were produced but also a new type of rays were produced simultaneoulsy from anode moving towards cathode and passes through the holes or canal of the cathode. These termed as canal rays or anode rays. These rays travel in straight lines and consists of positively charged particles. These rays have kinetic energy and produces heating effect also. The e/m ratio of these rays is smaller than that of electrons. Unlike cathode rays, their e/m value is dependent upon the nature of the gas taken in the tube. These rays produced flashes of light on ZnS screen and can pass throughs thin metal foils. They can produce physical and chemical changes and are capable to produce ionisation in gases. For cathode rays the value of e/m:
AAKASH INSTITUTE ENGLISH-ORGANIC CHEMISTRY: SOME BASIC PRINCIPLE AND TECHNIQUES-SECTION-I SUBJECTIVE TYPE QUESTIONS
- Rank the given species in the increasing order of water solubility
Text Solution
|
- Write the IUPAC names of the given compounds CH-=C-CH=CH-CH=CH2
Text Solution
|
- The number of possible alkynes with molecular formula C5H8 is
Text Solution
|
- HC-=CHoverset("Excess Na")rarrAoverset("Excess "CH(3)Cl)rarrB. The f...
Text Solution
|
- 'Stability of carbocations depends upon the electron releasing inducti...
Text Solution
|
- For the dehydration reaction.
Text Solution
|
- Which of the following is least stable ?
Text Solution
|
- Aromatic amines are weakly basic, whereas the below given compound is ...
Text Solution
|