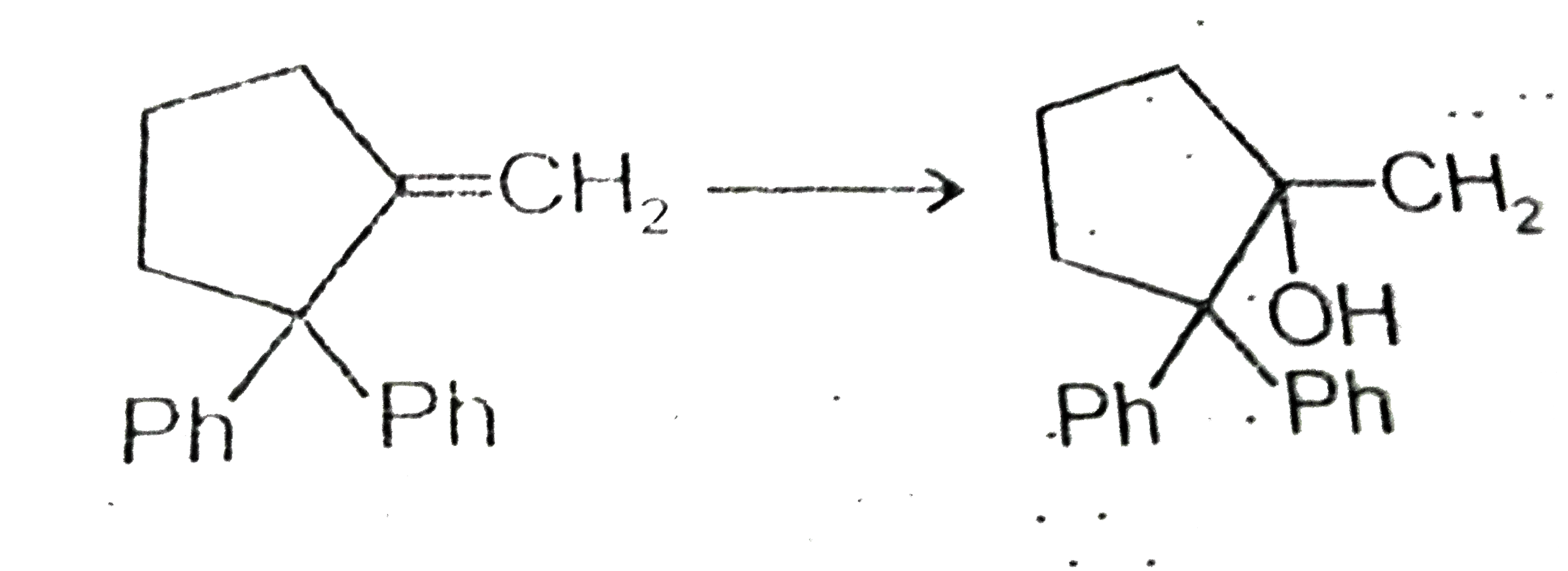
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
HYDROCARBONS
AAKASH INSTITUTE ENGLISH|Exercise SECTION-E ASSERTION-REASON TYPE QUESTIONS:|9 VideosHYDROCARBONS
AAKASH INSTITUTE ENGLISH|Exercise SECTION-F MATRIX-MATCH TYPE QUESTIONS|5 VideosHYDROCARBONS
AAKASH INSTITUTE ENGLISH|Exercise SECTION-C OBJECTIVE TYPE QUESTIONS (MORE THAN ONE OPTIONS ARE CORRECT)|12 VideosHALOALKANES AND HALOARENES
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT SECTION -D|15 VideosHYDROGEN
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (SECTION - D) (Assertion-Reason Type question)|15 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-HYDROCARBONS-SECTION-D LINKED COMPREHENSION TYPE QUESTIONS
- It we see the reaction of methane with halogen, the rate determining s...
Text Solution
|
- If we see the reaction of methane with halogen, the rate determining s...
Text Solution
|
- Addition of water molecule across double bond to yield Antimarkownikov...
Text Solution
|
- Addition of water molecule across double bond to yield Antimarkownikov...
Text Solution
|
- Addition of water molecule across double bond to yield Antimarkownikov...
Text Solution
|
- Hydration reaction of alkene is catalyzed by dilute acid. Selection of...
Text Solution
|
- Hydration reaction of alkene is catalyzed by dilute acid. Selection of...
Text Solution
|