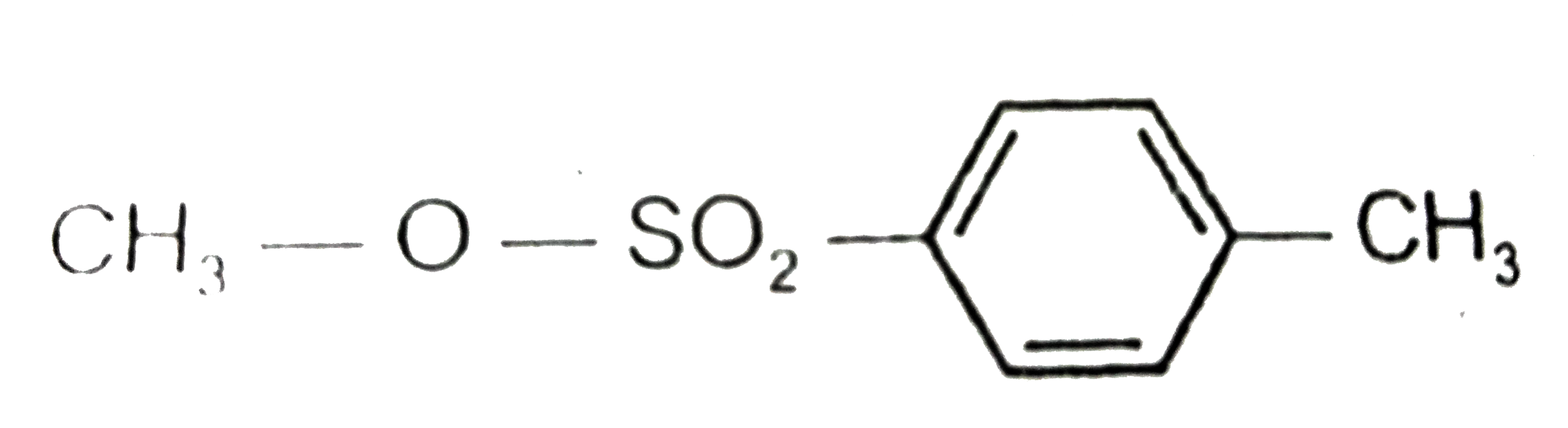
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HALOALKANES AND HALOARENES
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section - C ) ( Objective Type Question(More than one options are correct ))|17 VideosHALOALKANES AND HALOARENES
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section - D) (Linked Comprehension Type Question )|8 VideosHALOALKANES AND HALOARENES
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section - A ) ( Competition Level Question )|50 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
AAKASH INSTITUTE ENGLISH|Exercise Try Yourself|33 VideosHYDROCARBONS
AAKASH INSTITUTE ENGLISH|Exercise Assignment(Section - C) (Previous Years Questions)|60 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-HALOALKANES AND HALOARENES -Assignment (Section - B ) ( Objective Type Question(One option is correct ))
- 3 methyl -2- pentene on reaction with HOCI gives
Text Solution
|
- Among the three possible isomers of dibromo benzenes the highest ...
Text Solution
|
- Benzal chloride on hydrolysis gives
Text Solution
|
- For the reaction R- Br to R- O - N =O the suitable reagent is
Text Solution
|
- Compound X in the reaction is
Text Solution
|
- What would be the major product of the given reaction ?
Text Solution
|
- Arrange the given alkylhalides in the increasing reactivity towar...
Text Solution
|
- Which of the following substrate is most reactive towards methoxi...
Text Solution
|
- Each of the following reaction is given by tertiary butylbromide...
Text Solution
|
- On the basis of the given reaction sequence find out the final...
Text Solution
|
- Which of the following compound will given yellow precipitate on ...
Text Solution
|
- When chloroform reacts with NaOH an important reactive intermedi...
Text Solution
|
- Through S(N)2 reaction we cannot convert
Text Solution
|
- Suppose that CH(3) CH(2) I is added to an enthanol solution contain...
Text Solution
|
- Rank the following compounds in order of increasing E(2) reaction ...
Text Solution
|
- PhMgBr (Grignard reagent ) cannot be used to prepare the compound ...
Text Solution
|
- Which chloroderivative of nitrobenzene among the following would...
Text Solution
|
- A chemist plans to prepare 1- bromo-2- pentene by the following re...
Text Solution
|
- The two compounds shown in the figure below are
Text Solution
|
- The reaction of phenylacetylene with one equivalent of methyl magnes...
Text Solution
|