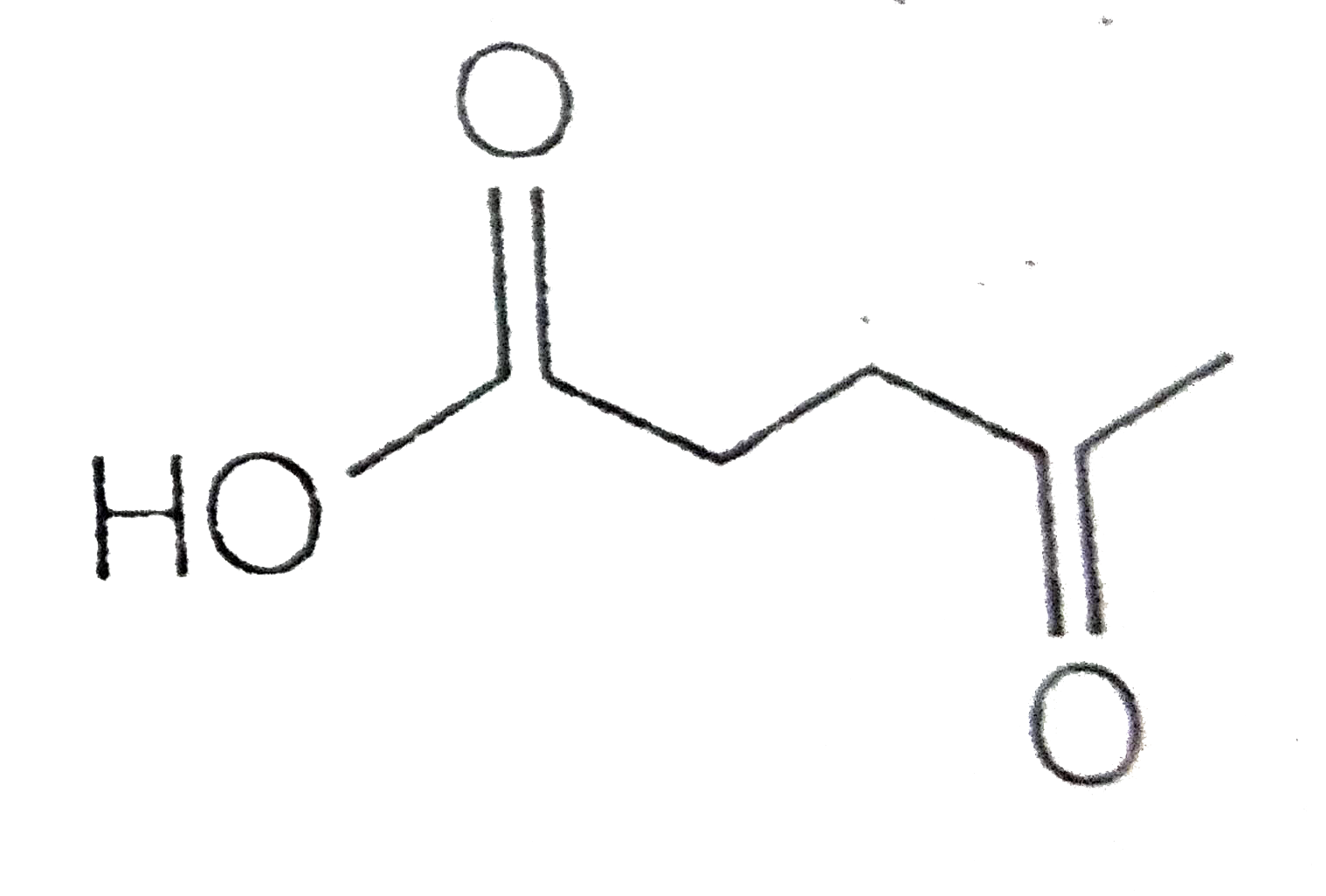
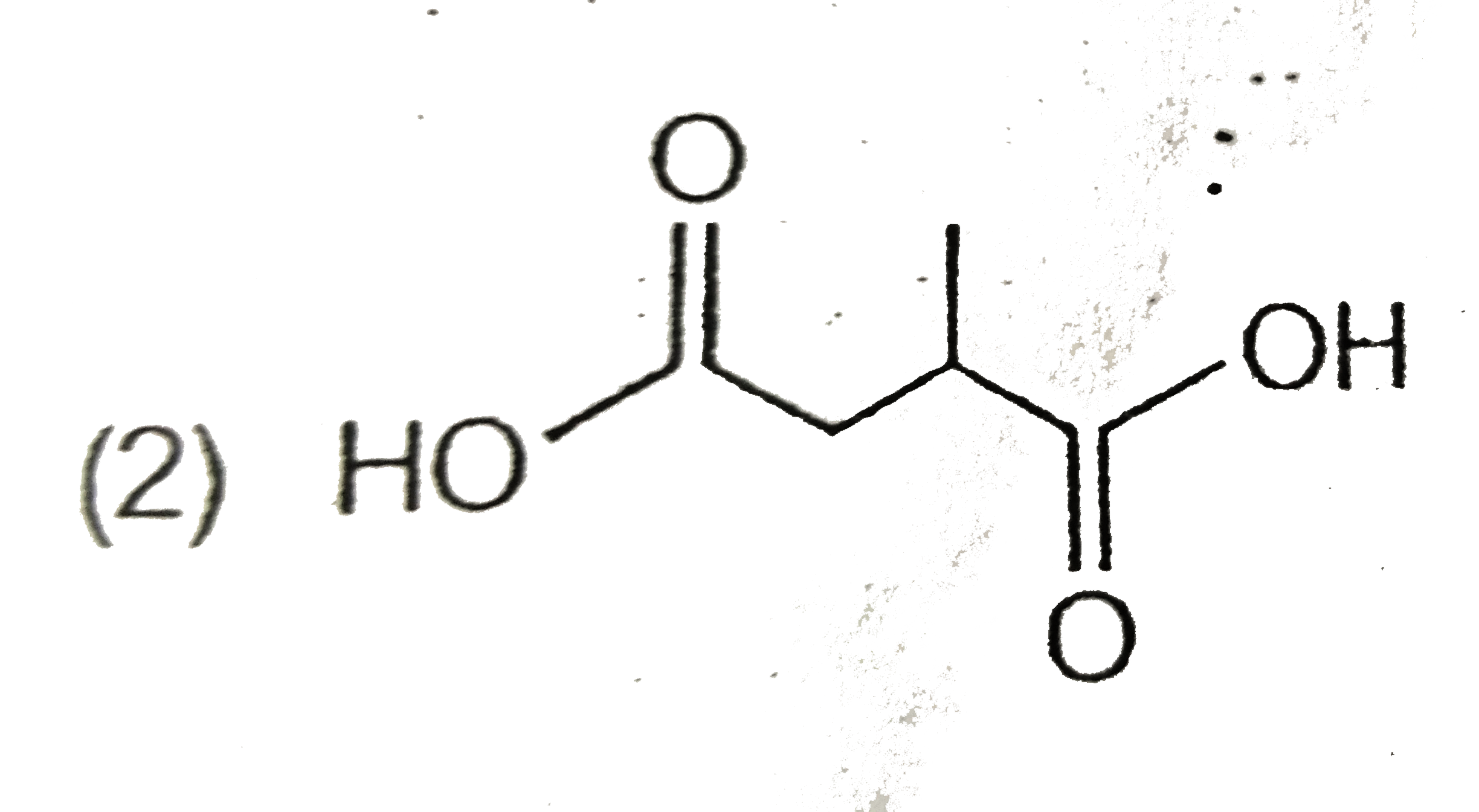
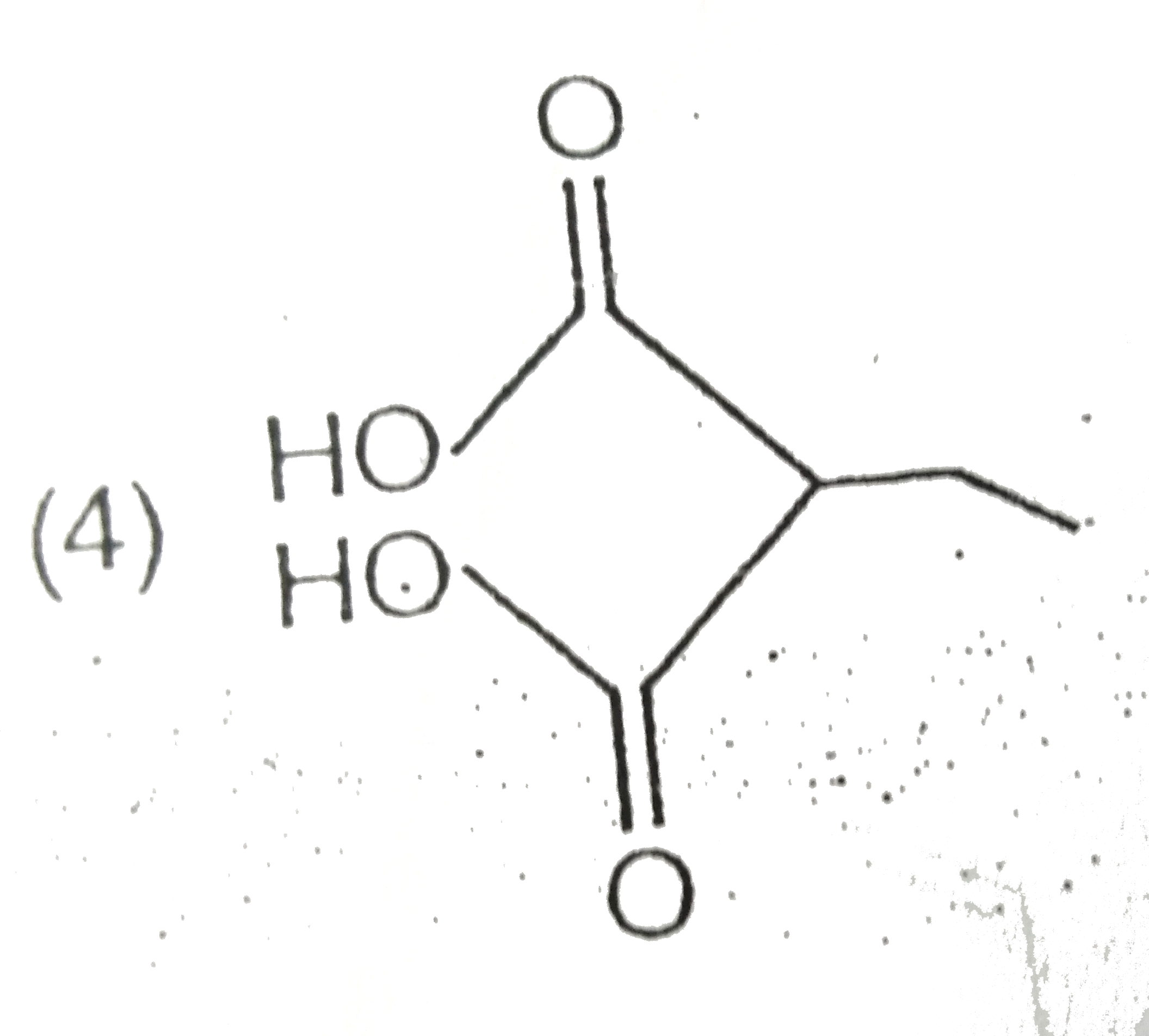
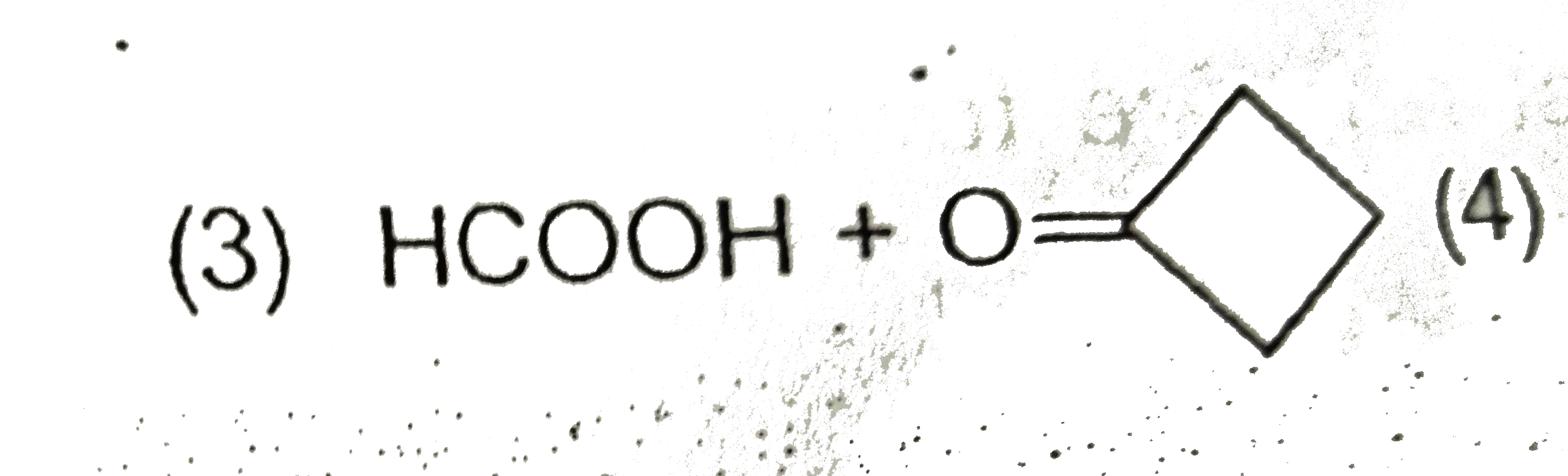A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HALOALKANES AND HALOARENES
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section - E) ( Assertion - Reason type Questions )|7 VideosHALOALKANES AND HALOARENES
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section - F) (Matrix - Match Type Question )|4 VideosHALOALKANES AND HALOARENES
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section - C ) ( Objective Type Question(More than one options are correct ))|17 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
AAKASH INSTITUTE ENGLISH|Exercise Try Yourself|33 VideosHYDROCARBONS
AAKASH INSTITUTE ENGLISH|Exercise Assignment(Section - C) (Previous Years Questions)|60 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-HALOALKANES AND HALOARENES -Assignment (Section - D) (Linked Comprehension Type Question )
- Compound which rotates the plane polarised light is known as op...
Text Solution
|
- Which of the following molecule can resolved into enantiomers ?
Text Solution
|
- A compound (A) has molecular formula C(5)H(9)CI It does not rea...
Text Solution
|
- A compound (A) has molecular formula C(5)H(9)CI It does not rea...
Text Solution
|
- A compound (A) has molecular formula C(5)H(9)Cl .It does not re...
Text Solution
|
- Bacause of the resonance stabilization of Arylhalides they are un...
Text Solution
|
- Bacause of the resonance stabilization of Arylhalides they are un...
Text Solution
|
- Bacause of the resonance stabilization of Arylhalides they are un...
Text Solution
|