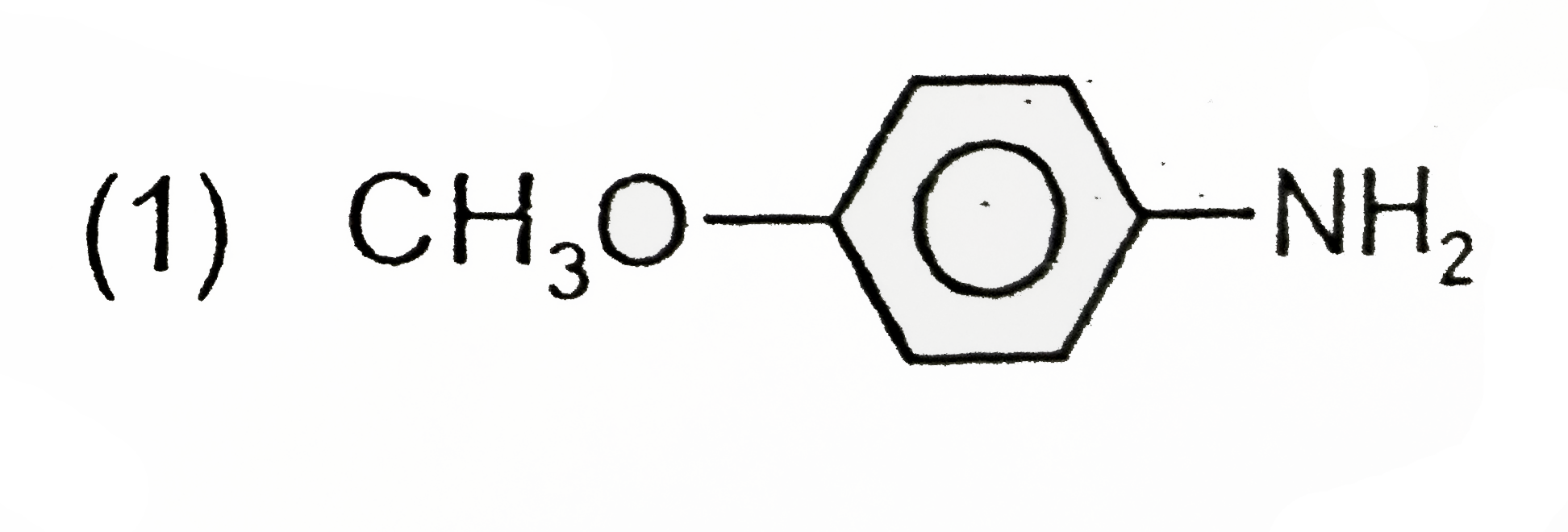
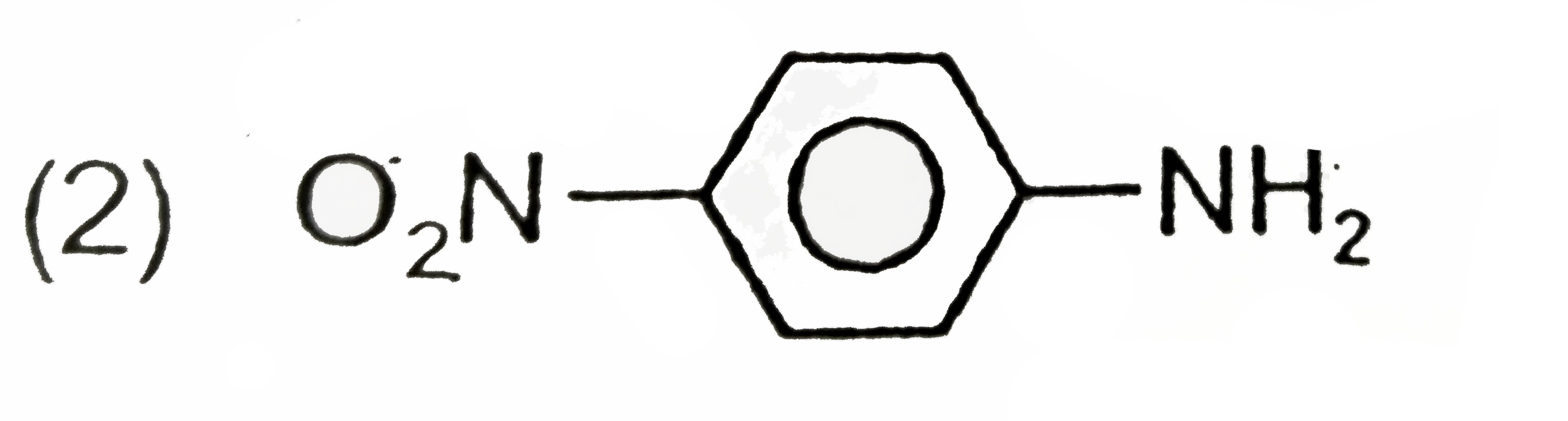
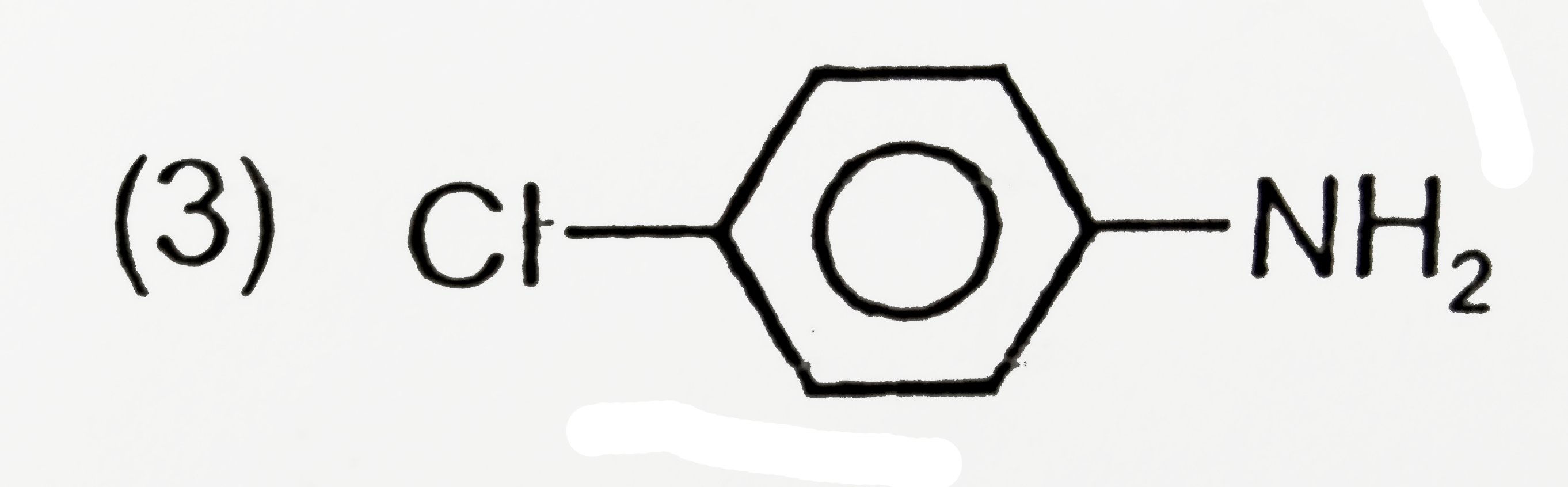
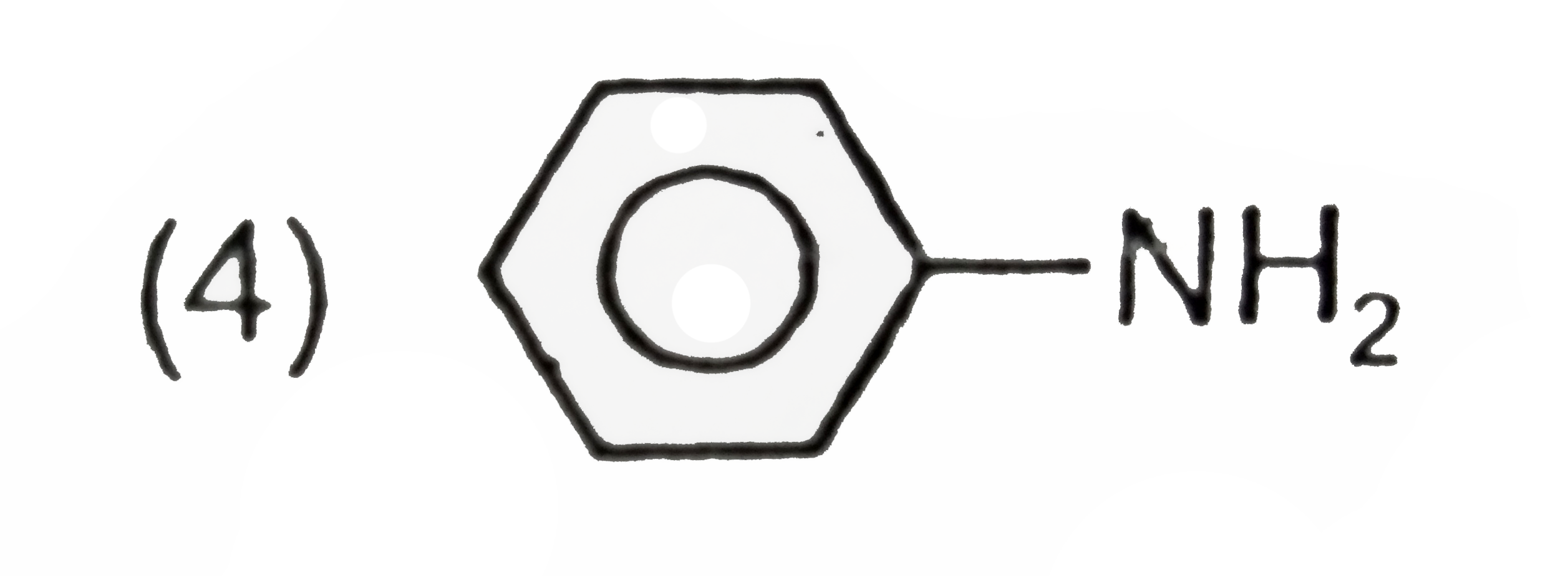
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
AMINES
AAKASH INSTITUTE ENGLISH|Exercise Assignment Section C (Objective type questions more than one options are correct)|12 VideosAMINES
AAKASH INSTITUTE ENGLISH|Exercise Assignment Section D Linked Comprehension Type Questions|15 VideosAMINES
AAKASH INSTITUTE ENGLISH|Exercise Assignment Section -A Competition Level Questions|50 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
AAKASH INSTITUTE ENGLISH|Exercise Try Yourself|20 VideosBIOMOLECULES
AAKASH INSTITUTE ENGLISH|Exercise EXERCISE (ASSIGNMENT) SECTION - D Assertion - Reason Type Questions|5 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-AMINES -Assignment Section B (Objective Type Questions) one option is correct
- Which of the following reagent converts nitrobenzene to aniline?
Text Solution
|
- Which of the following is most basic?
Text Solution
|
- Which of the following give isocyanide test?
Text Solution
|
- Which of the following give para major product with aniline?
Text Solution
|
- The product C is
Text Solution
|
- The product is
Text Solution
|
- The incorrect statement among the following is
Text Solution
|
- A mixture containing primary, secondary and tertiary amine is treated ...
Text Solution
|
- The compound A and B are:
Text Solution
|
- CH(3)CH(2)NH(2) overset((CH(3)CO)(2)O)underset(Delta) to A+B, (A) and ...
Text Solution
|
- Choose the false statement
Text Solution
|
- End product of the given reaction sequence is
Text Solution
|
- Among the following compounds which one will produce an enamine on rea...
Text Solution
|
- An optically active compound [A] C(5)H(13)N reacts with alkaline CHCl(...
Text Solution
|
- Final product of the following sequence of reactions would be
Text Solution
|
- Methyl orange (an acid-base indicator) can be prepared by following se...
Text Solution
|
- Consider the following sequence of reactions: [P] gives coloured ...
Text Solution
|
- Many important pencilin type antibiotics possess a beta-lactum unit. W...
Text Solution
|
- A nitrogenous compound (X) is treated with HNO(2), and the mixture is ...
Text Solution
|
- Consider the following sequence of reaction The product [P] would...
Text Solution
|