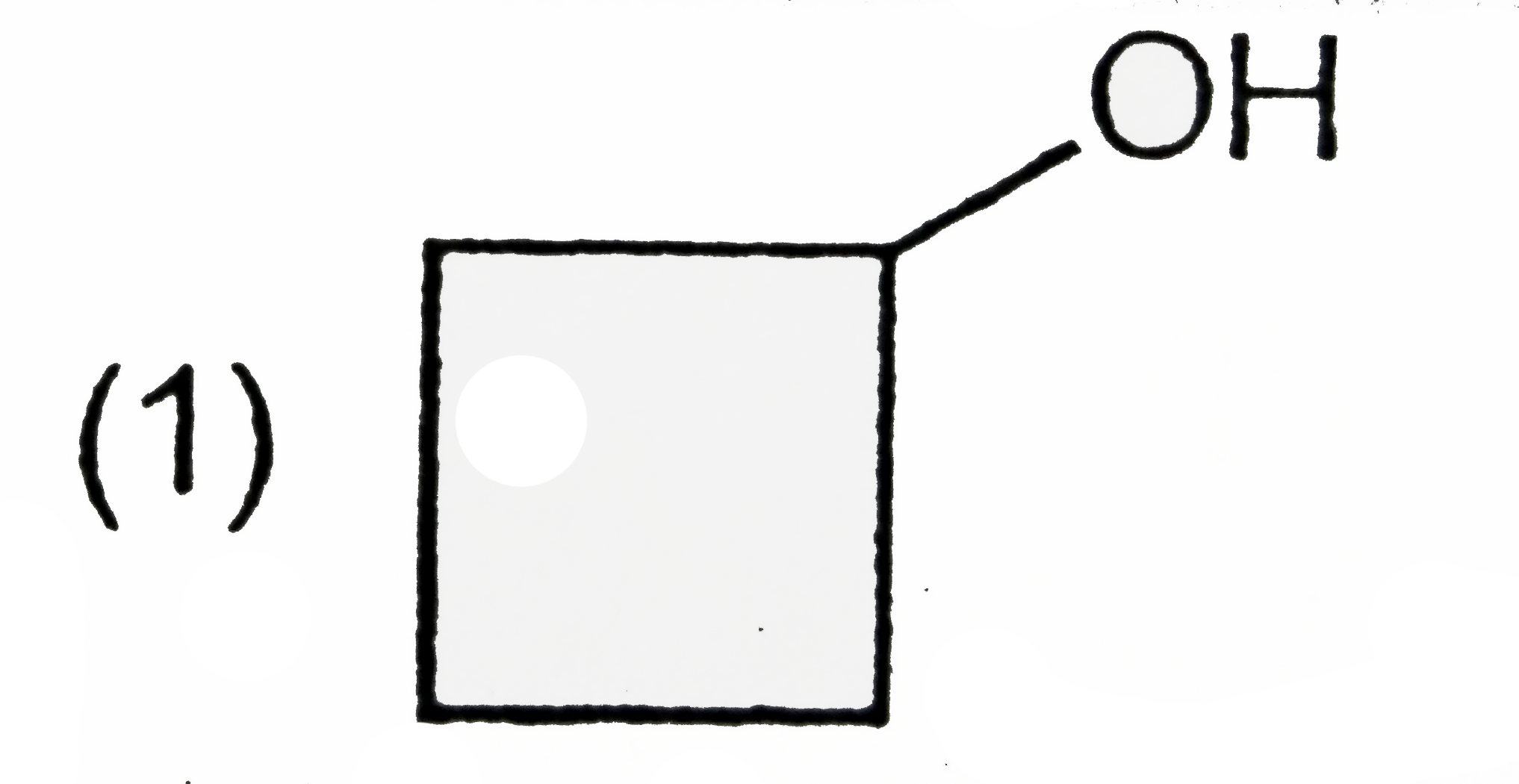
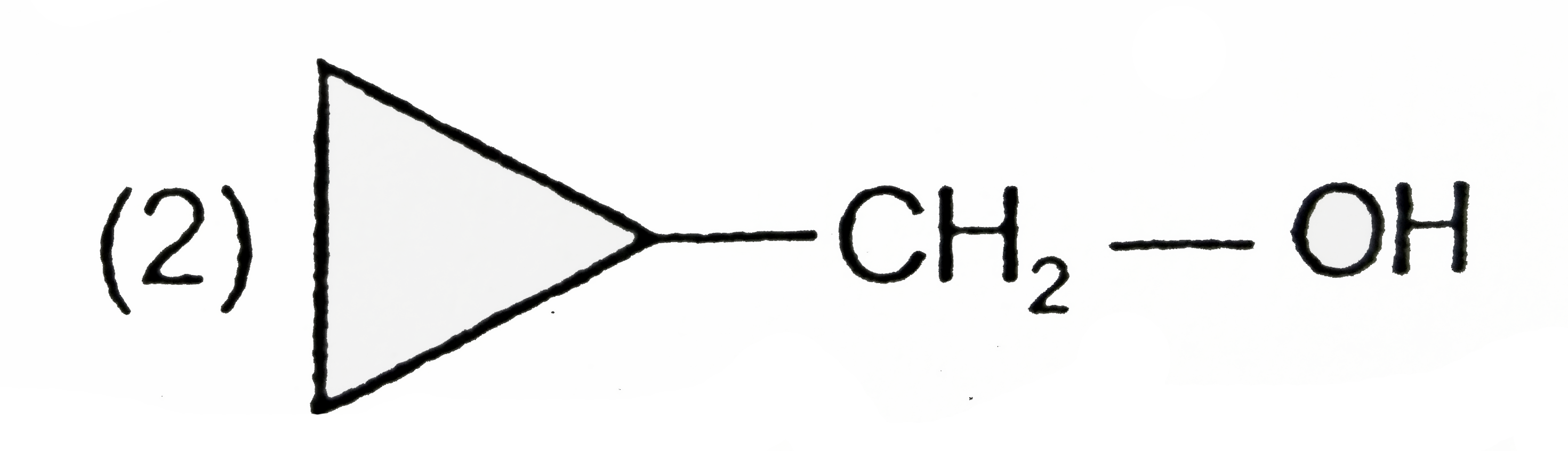
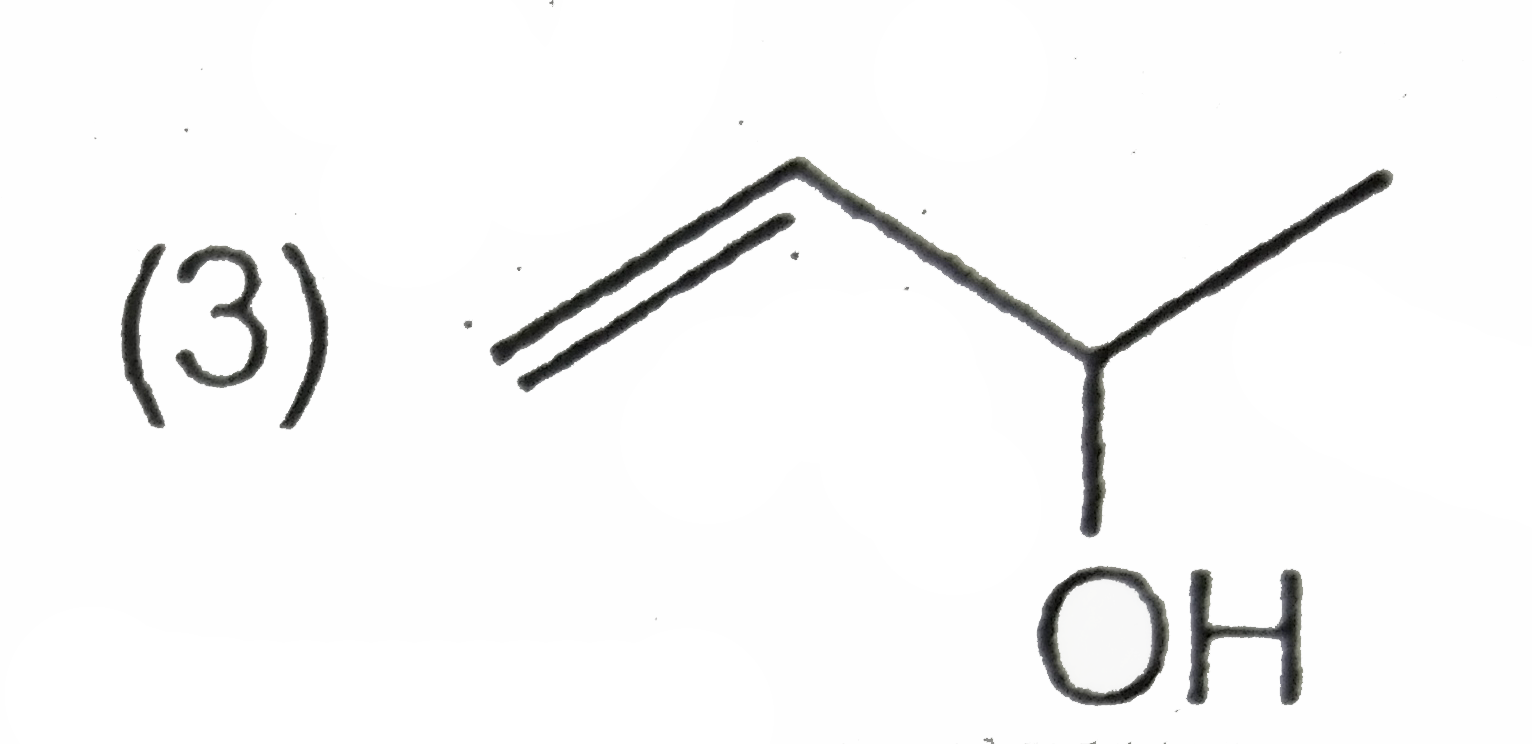
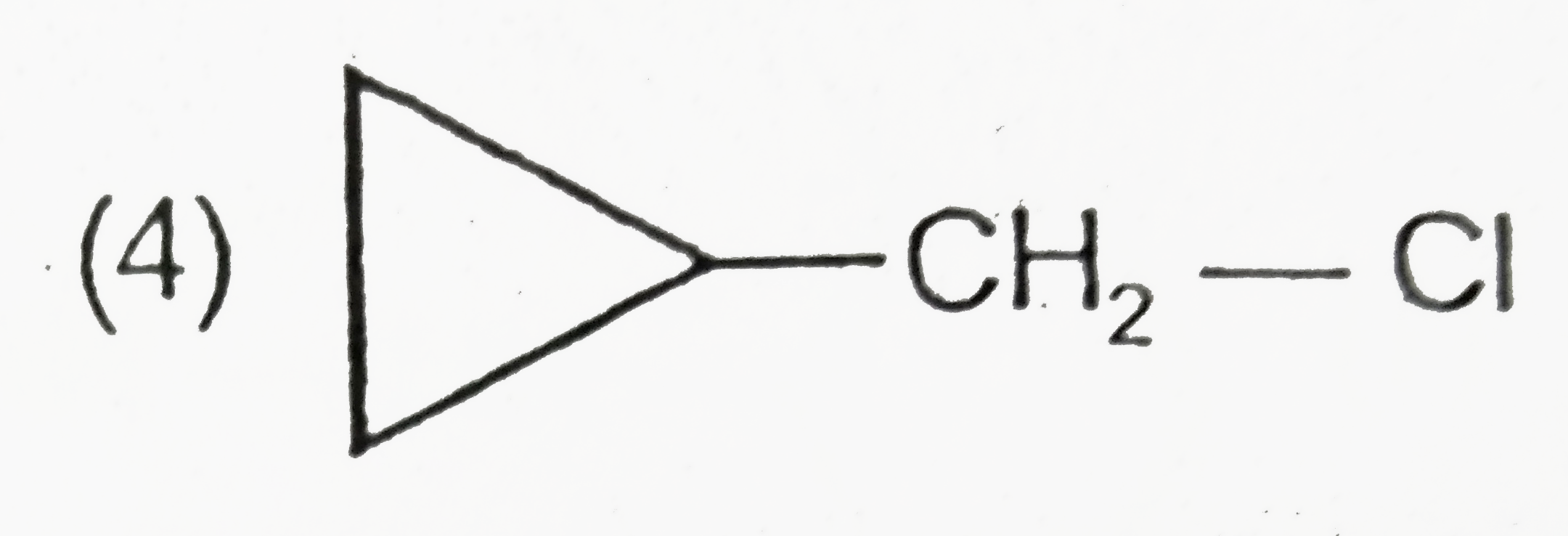
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
AMINES
AAKASH INSTITUTE ENGLISH|Exercise Assignment Section D Linked Comprehension Type Questions|15 VideosAMINES
AAKASH INSTITUTE ENGLISH|Exercise Assignment Section E Assertion-Reason Type Questions|9 VideosAMINES
AAKASH INSTITUTE ENGLISH|Exercise Assignment Section B (Objective Type Questions) one option is correct|23 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
AAKASH INSTITUTE ENGLISH|Exercise Try Yourself|20 VideosBIOMOLECULES
AAKASH INSTITUTE ENGLISH|Exercise EXERCISE (ASSIGNMENT) SECTION - D Assertion - Reason Type Questions|5 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-AMINES -Assignment Section C (Objective type questions more than one options are correct)
- Which of the following compounds on hydrolysis yields a carboxylic aci...
Text Solution
|
- Which of the following reactions are suitable method for the preparati...
Text Solution
|
- Consider the following reaction The product [P] is known as
Text Solution
|
- Cyclobutyl amine is treated with sodiumnitrite and aqueous HCl. The pr...
Text Solution
|
- Which of the following reagents can be used to distinguish primary ami...
Text Solution
|
- Which of the following reactions give the poor yield of amine products...
Text Solution
|
- Which of the following order of basicity is correct in aqueous medium?
Text Solution
|
- What would be the final products of the given sequence of reaction?
Text Solution
|
- Which of the following does not undergo diazotization reaction?
Text Solution
|
- Which of the following compounds do not give Friedel Crafts reaction?
Text Solution
|
- -NH(2) group is a strong activator towards aromatic electrophilic subs...
Text Solution
|
- Identify the end product in the reaction
Text Solution
|