Text Solution
Verified by Experts
Topper's Solved these Questions
NUCLEI
AAKASH INSTITUTE ENGLISH|Exercise Try Yourself|36 VideosNUCLEI
AAKASH INSTITUTE ENGLISH|Exercise Assignment Section A Objective (One option is correct )|52 VideosMOVING CHARGES AND MAGNETISM
AAKASH INSTITUTE ENGLISH|Exercise Assignment Section J (Aakash Challengers Questions)|5 VideosOSCILLATIONS
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section D) (ASSERTION-REASON TYPE QUESTIONS)|13 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-NUCLEI-ASSIGNMENT (SECTION-D)
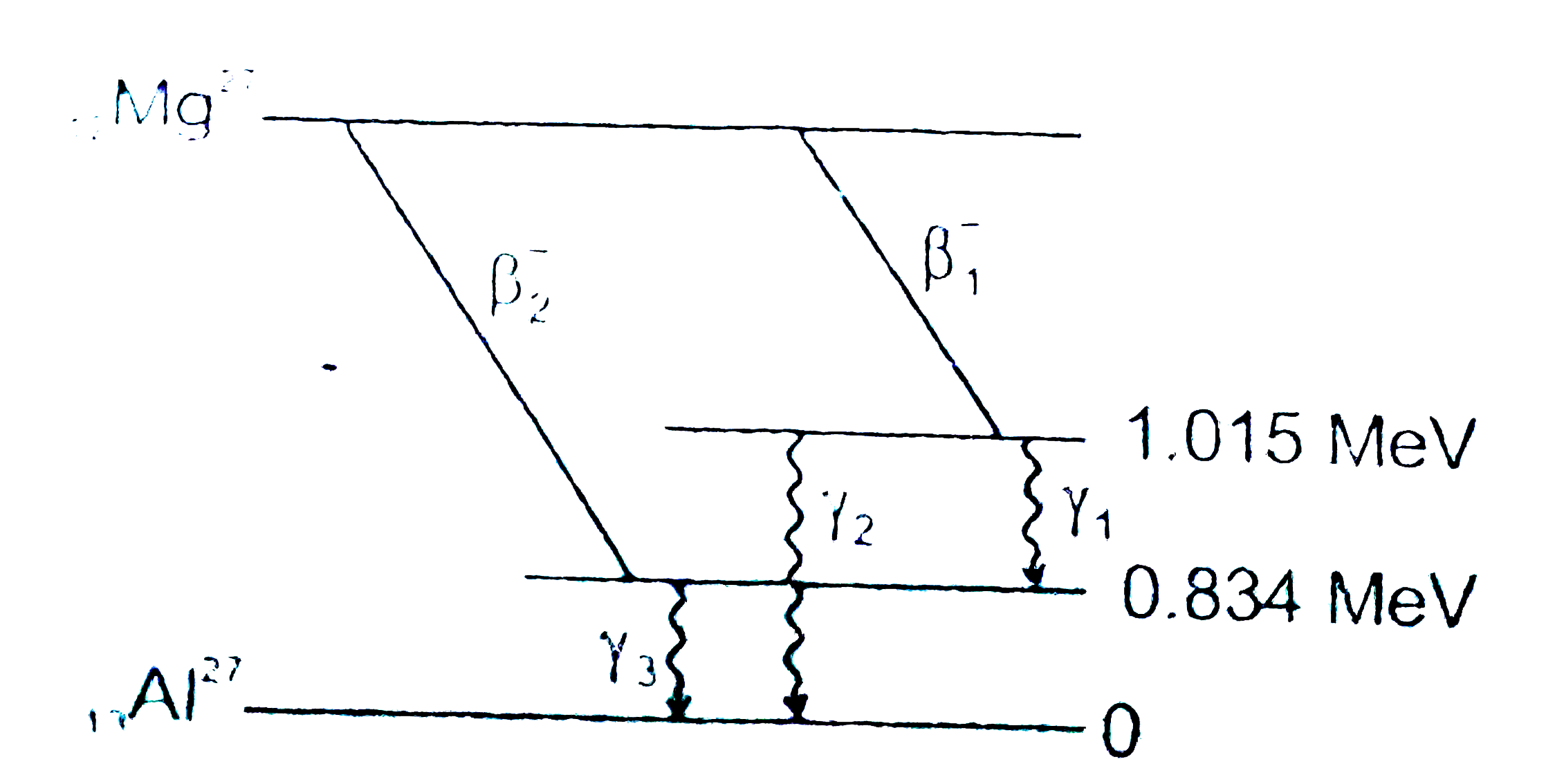
- Calculate the kinetic energy of beta-particles and the radiation frequ...
Text Solution
|
- A : Uncertainty principle demands that an electron confined to a nucle...
Text Solution
|
- A: A free proton is stable but inside a nucleus , a proton gets conver...
Text Solution
|
- A : Exothermic reactions are possible when two light nuclei fuse or wh...
Text Solution
|
- A : For fusion, the light nuclei must have sufficient initial energy t...
Text Solution
|
- Atomic number is the sum of number of protons and neutrons.
Text Solution
|
- A : Nuclear density is almost same for all nuclei . R: The radius (r...
Text Solution
|
- A : During radioactive disintegration an alpha-particle and a beta-par...
Text Solution
|
- A: In beta-decay an electron is emitted by the nucleus R: Electrons ...
Text Solution
|
- A: A radioactive substance has half life of 4 hour. Therefore, if two ...
Text Solution
|
- A : Fast moving neutrons do not cause fission of a uranium nucleus. ...
Text Solution
|