Text Solution
Verified by Experts
Topper's Solved these Questions
PERMUTATIONS AND COMBINATIONS
AAKASH INSTITUTE ENGLISH|Exercise Assignment Section-I (Subjective Type Questions)|11 VideosMATRICES
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section - J) Aakash Challengers Questions|3 VideosPRINCIPLE OF MATHEMATICAL
AAKASH INSTITUTE ENGLISH|Exercise Section-D:(Assertion-Reason Type Questions)|11 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-PERMUTATIONS AND COMBINATIONS -Assignment Section-J (Aakash Challengers Questions)
- The symbols + , + , xx , xx , *** ,cdot are placed in the squares of ...
Text Solution
|
- The number of triangles that can be formed form a regular polygon of 2...
Text Solution
|
- The total number of non- similar triangles which can be formed such th...
Text Solution
|
- On the modified chess board 10 xx 10. Amit and Suresh two persons whic...
Text Solution
|
- Determine the number of ordered pairs of positive integers (a , b) suc...
Text Solution
|
- Find the number of ways in which we can choose 3 squares on la ches...
Text Solution
|
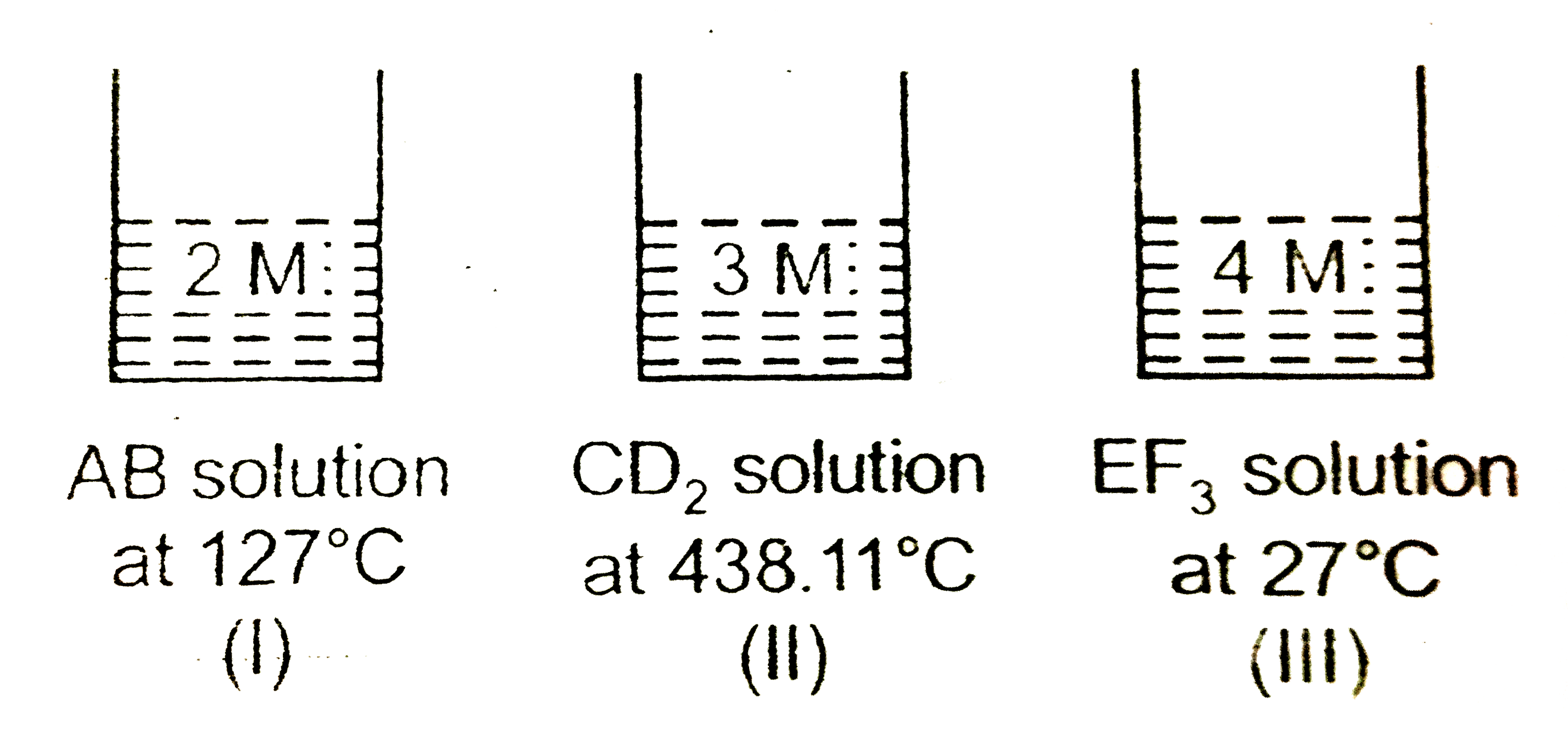
- Consider three solutions of 3 strong electrolytes.AB, CD(2) and EF(3) ...
Text Solution
|