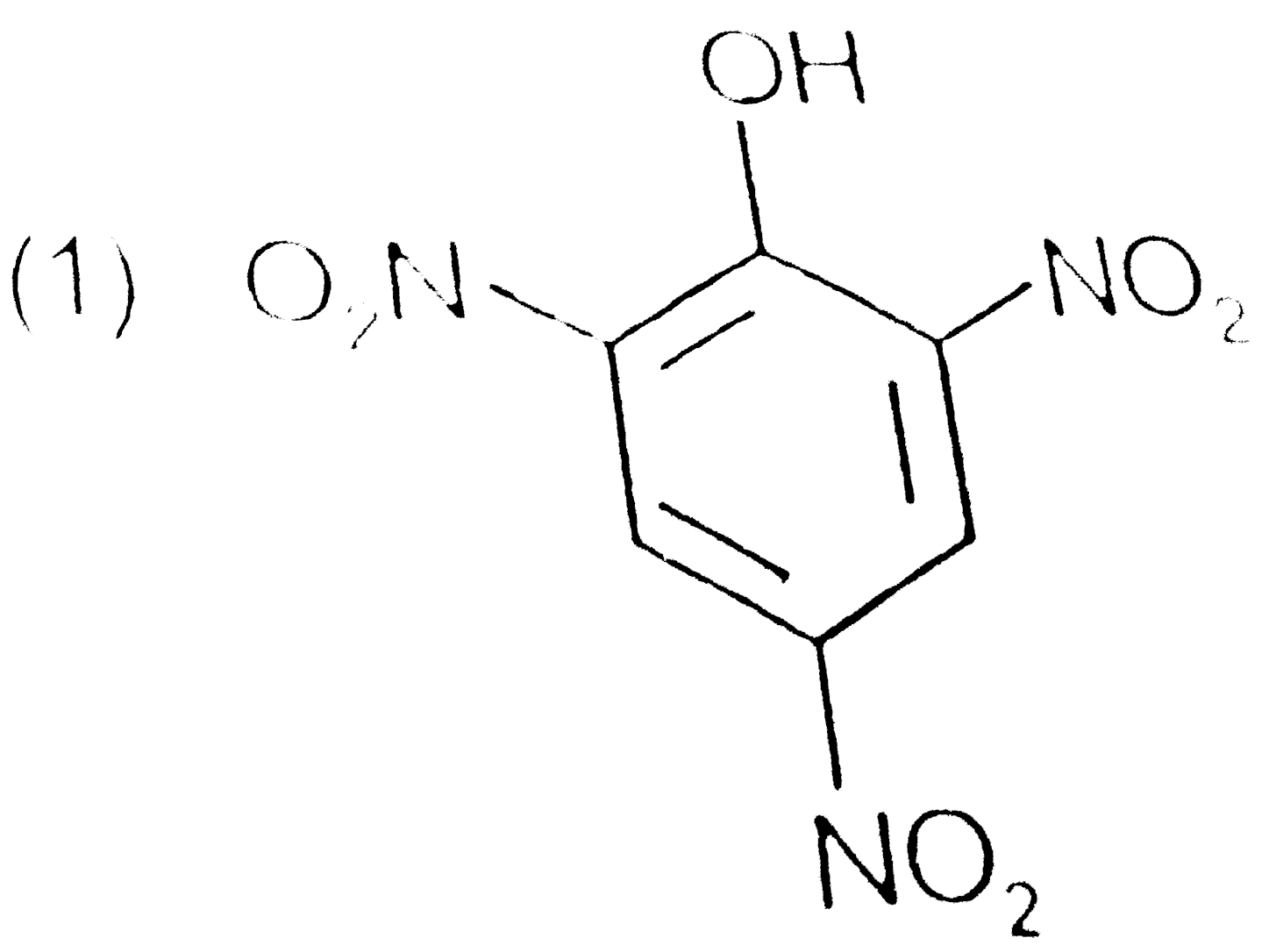
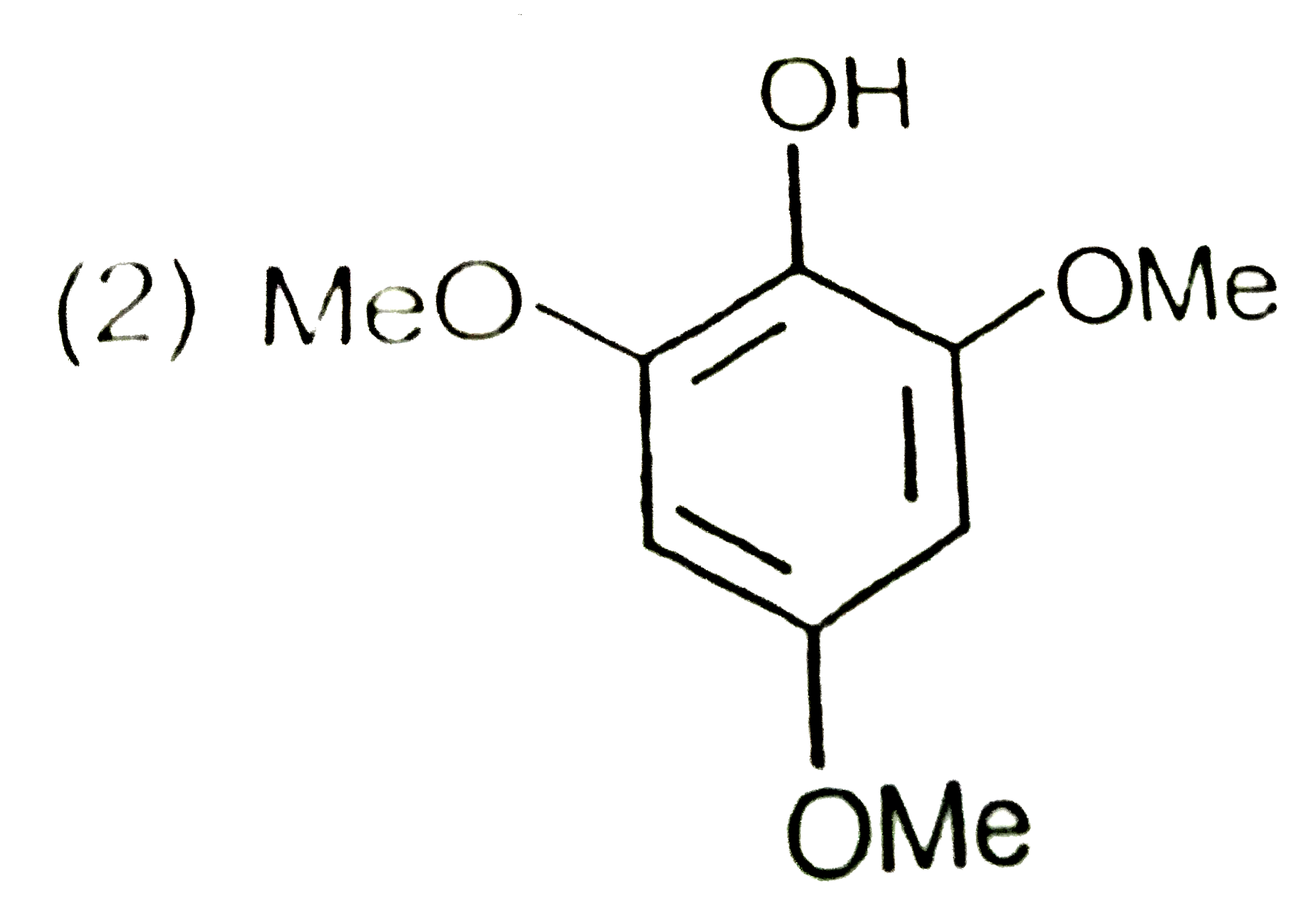
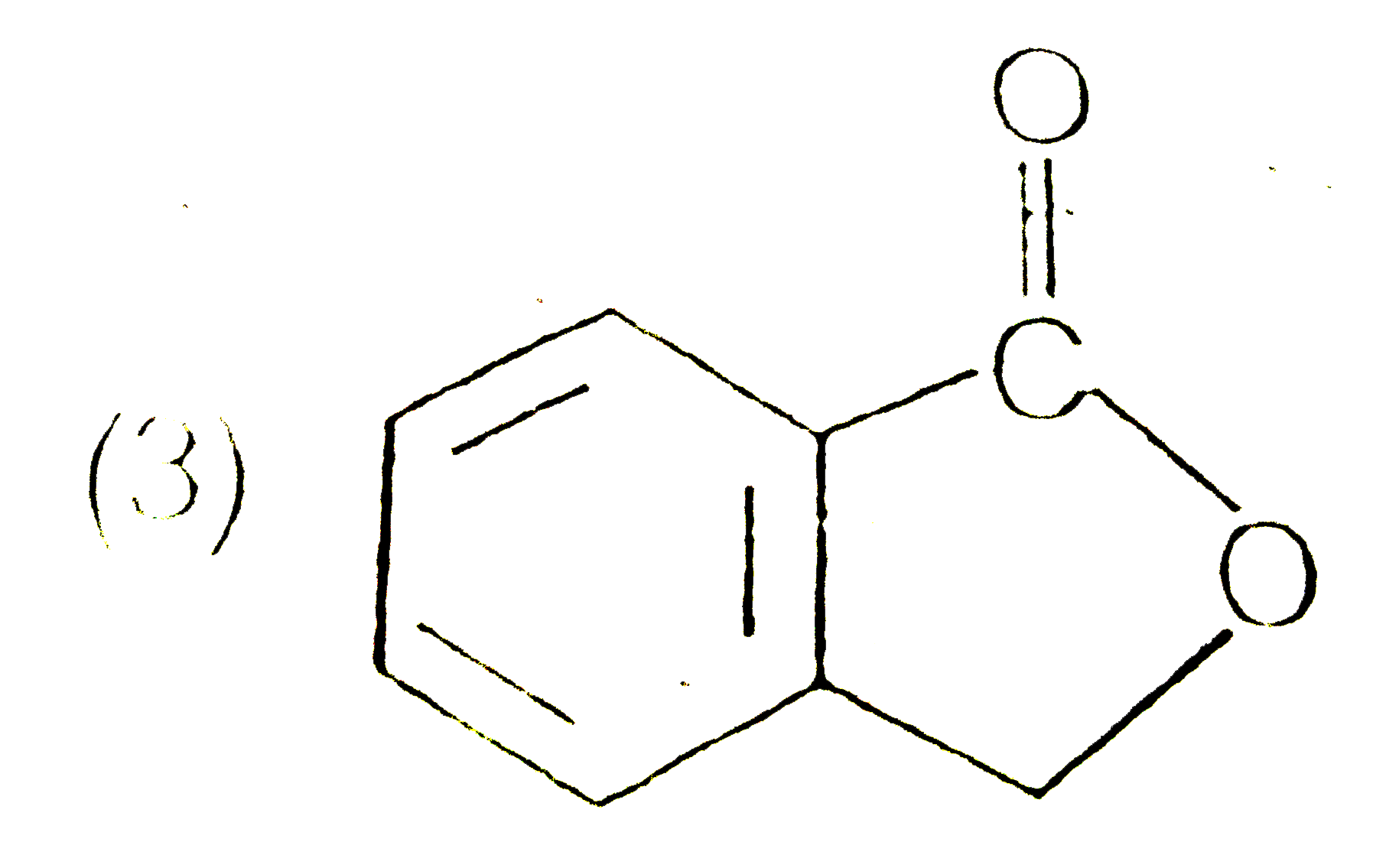
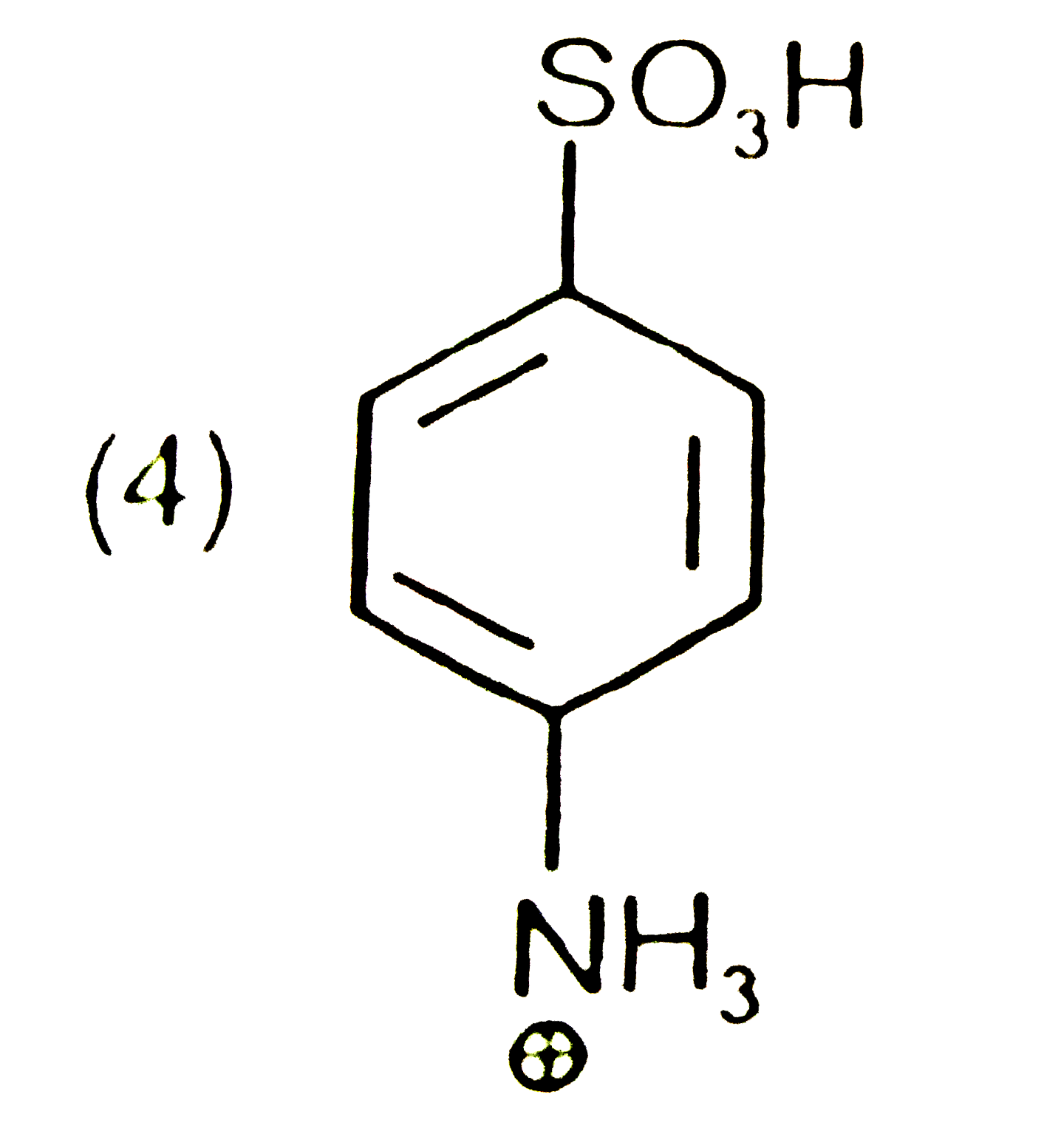
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ALDEHYDES, KETONES AND CARBOXYLIC ACIDS
AAKASH INSTITUTE ENGLISH|Exercise Assignment (SECTION -D Linked Comprehension type Questions)|6 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
AAKASH INSTITUTE ENGLISH|Exercise Assignment SECTION - E Assertion - Reason Type Questions)|7 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
AAKASH INSTITUTE ENGLISH|Exercise Assignment (SECTION -B OBJECTIVE TYPES QUESTIONS (ONE OPTION IS CORRECT)|24 VideosALCOHOLS, PHENOLS AND ETHERS
AAKASH INSTITUTE ENGLISH|Exercise Try Yourself|5 VideosAMINES
AAKASH INSTITUTE ENGLISH|Exercise Assignment Section -J (Aakash Challengers Questions)|9 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-ALDEHYDES, KETONES AND CARBOXYLIC ACIDS -Assignment (SECTION -C Objective type questions more than one options are correct)
- Which of the following reactions involve carbanion enolalte as reactiv...
Text Solution
|
- Which of the following compounds can be synthesized by intramolecular ...
Text Solution
|
- Which of the following dicarboxylic acid will give cycle alkanone on h...
Text Solution
|
- Which of the following compounds will give over all substitution produ...
Text Solution
|
- Which of the following reagents can be used to distinguish Benzaldehdy...
Text Solution
|
- Which of the following compounds could liberate CO(2) with aqueous NaH...
Text Solution
|
- Which of the following intermediates are involved in the acid catalyze...
Text Solution
|
- Consider the following sequence of reactions Products of the give...
Text Solution
|
- Which of the following compounds will give tertiary butanol as the maj...
Text Solution
|
- Which of the following statements are correct regarding given reaction...
Text Solution
|
- Identify the set of reagents/ reaction conditions X and Y in the follo...
Text Solution
|