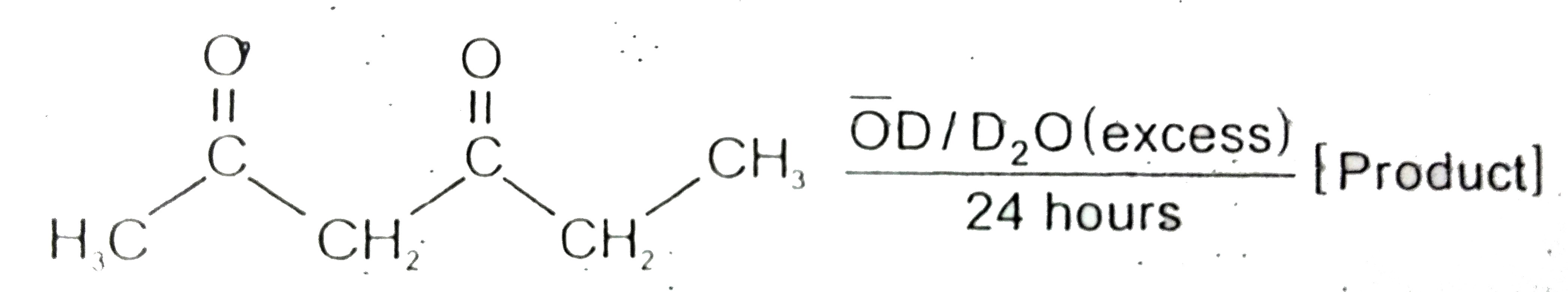
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALDEHYDES, KETONES AND CARBOXYLIC ACIDS
AAKASH INSTITUTE ENGLISH|Exercise Assignment (SECTION -H Multiple True-False Type Questions)|2 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
AAKASH INSTITUTE ENGLISH|Exercise Assignment (SECTION -I Subjective Type Questions)|10 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
AAKASH INSTITUTE ENGLISH|Exercise Assignment (SECTION - F Matrix Match type Questions)|4 VideosALCOHOLS, PHENOLS AND ETHERS
AAKASH INSTITUTE ENGLISH|Exercise Try Yourself|5 VideosAMINES
AAKASH INSTITUTE ENGLISH|Exercise Assignment Section -J (Aakash Challengers Questions)|9 Videos
Similar Questions
Explore conceptually related problems