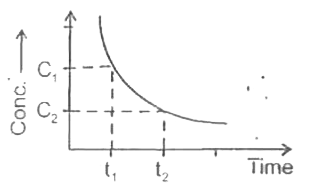
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (SECTION B : Objective Type Questions)|20 VideosCHEMICAL KINETICS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (SECTION C : Previous Year Questions)|62 VideosCHEMICAL KINETICS
AAKASH INSTITUTE ENGLISH|Exercise EXERCISE|30 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
AAKASH INSTITUTE ENGLISH|Exercise Assignment Section J (Aakash Challengers Questions)|10 VideosCHEMISTRY IN EVERYDAY LIFE
AAKASH INSTITUTE ENGLISH|Exercise Assignment ( SECTION - A)|45 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-CHEMICAL KINETICS-ASSIGNMENT (SECTION A : Objective Type Questions)
- In the reaction , A+2Bto6C+2D, if the initial rate -(d[A])/(dt) at t= ...
Text Solution
|
- For the reaction N(2) + 3 H(2) to 2 NH(3) The rate of change of conc...
Text Solution
|
- The graph plotted between concentration versus time
Text Solution
|
- For reaction, 2B+A rarr 2C Which of the following is correct ?
Text Solution
|
- For the homogeneous elementary reaction, A+B rarrC, the unit of rate ...
Text Solution
|
- The rate law for a reaction between A and B is given by rate = k[A]^(n...
Text Solution
|
- Consider the reaction, 2A + B rarr C + D , If the rate expression is...
Text Solution
|
- The rate constant is numerically the same for three reaction of first ...
Text Solution
|
- Assuring an elementary reaction H2O2+3I^(-)+2H^(+)rarr2H2O+I3^(-) . Th...
Text Solution
|
- For a reaction A+B rarr products, the rate of reaction was doubled whe...
Text Solution
|
- The overall order of reaction between X & Y is 3. Which of the followi...
Text Solution
|
- For a zero order reaction, K=1xx10^(-3)"mol L"^(-1)s^(-1) If initi...
Text Solution
|
- Which of the following statement is not correct ?
Text Solution
|
- Consider the following in respect of zero order reaction. I. t(1//2)...
Text Solution
|
- If initial concentration is reduced to 1//4^(th) in a zero order react...
Text Solution
|
- If initial concentration is doubled, the time for half reaction is als...
Text Solution
|
- The inversion of cane sugar is represented by C(12)H(22)O(11) + H(2)...
Text Solution
|
- For a lst order reaction , a straight line is obtained if you plot
Text Solution
|
- Which order reaction obeys the expression t(1//2)prop1[A] ?
Text Solution
|
- The graph of t(1//2) versus initial concentration 'a' is for
Text Solution
|