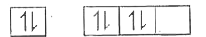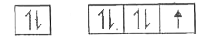A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
STRUCTURE OF ATOM
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (SECTION -B) Objective type questions|34 VideosSTRUCTURE OF ATOM
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (SECTION -C) Previous Years Questions|47 VideosSTRUCTURE OF ATOM
AAKASH INSTITUTE ENGLISH|Exercise EXERCISE|40 VideosSTATES OF MATTER
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (SECTION-D)|14 VideosSTRUCTURE OF ATOM
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT ( SECTION -J) Aakash Challengers Questions|12 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-STRUCTURE OF ATOM-ASSIGNMENT (SECTION -A) Objective type questions
- Arrange the following orbitals of H-atom in the increasing order of th...
Text Solution
|
- Which of the following set of quantum number is possible ?
Text Solution
|
- The maximum number of electrons in an atom which can have n=4 i...
Text Solution
|
- An orbital is described with the help of a wave function. Since many w...
Text Solution
|
- Assuming the velocity to be same , the wavelength of the waves ...
Text Solution
|
- For a 'p' electron the orbial angular momentum is
Text Solution
|
- Which electronic level would allow the hydrogen atom to absorb a photo...
Text Solution
|
- Which of the following transition will emit maximum energy in hyd...
Text Solution
|
- In an atom which has 2K, 8L , 18 M and 2N electrons in the ...
Text Solution
|
- The prodensity curve for 2s electrons appears like
Text Solution
|
- Any p-orbital can accommodate upto :
Text Solution
|
- If the uncertainty in the position of an electron is zero the uncerta...
Text Solution
|
- The number of radial nodes in 4s and 3p orbitals are respecti...
Text Solution
|
- Which of the following orbital is with the four lobes present o...
Text Solution
|
- Which of the following statements concerning the four quantum numbers ...
Text Solution
|
- Which of the following has maximum number of unpaired electrons...
Text Solution
|
- The two electrons in sub-shell of K-shell will differ in:
Text Solution
|
- Which of the following electronic configuration is not possible ...
Text Solution
|
- The orbital diagram in which both the pauli's exclusion principal and ...
Text Solution
|
- Which of the following electronic configuration is incorrect ?
Text Solution
|