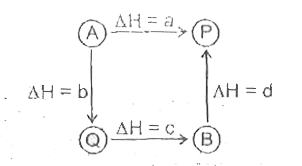
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (Section - A) Objective Type Questions|55 VideosTHERMODYNAMICS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (Section - B) Objective Type Questions|35 VideosTHERMODYNAMICS
AAKASH INSTITUTE ENGLISH|Exercise SECTION-J|7 VideosTHE SOLID STATE
AAKASH INSTITUTE ENGLISH|Exercise Assignment (SECTION - D) (ASSERTION-REASON TYPE QUESTION)|20 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-THERMODYNAMICS-Exercise
- For one mole of a diatomic gas gamma is equal to
Text Solution
|
- The enthalpy of reaction is maximum in which of the following reaction...
Text Solution
|
- On the basis of Hess's law of constant heat summation, choose the corr...
Text Solution
|
- In which of the following Delta H = Delta U ?
Text Solution
|
- Heat of combustion of CH(4),C(2)H(4),C(2)H(6) are -890,-1411 and -1560...
Text Solution
|
- The heats of neutralisation of four acidsA,B,C and D are -13.7,-9.4,-1...
Text Solution
|
- Heat of neutralisation of NaOH and HCl is -57.46 kJ // equivalent. The...
Text Solution
|
- Bond energy of N-H,H-H and N-=N are a,b,c respectively. The DeltaH for...
Text Solution
|
- At 27^(@) C, the combustion of ethane takes place according to the rea...
Text Solution
|
- For the reaction PCI (3) (g) + CI(2)(g) rightarrow PCI(5)(g), Delta ...
Text Solution
|
- Heat of which of the following reaction gives enthalpy of formation di...
Text Solution
|
- When Delta H and TDeltaS both are negative , then for spontaneous pro...
Text Solution
|
- Which of the following relation is false?
Text Solution
|
- In a reversible process, the value of Delta S (sys) + Delta S(surr) is
Text Solution
|
- A boiled egg shows a/an ....... In entropy
Text Solution
|
- The Delta G in the process of melting of Ice at -15^(@) C is
Text Solution
|
- The spontaneous nature of a reaction is impossible if
Text Solution
|
- Delta G^(@) of reversible reaction at its equilbrium is
Text Solution
|
- Latent heat of fusion of ice is 0.333 kJ g^(-1) . The increase in entr...
Text Solution
|
- The value for DeltaH(vap) and Delta S(vap) for ethanol are respectivel...
Text Solution
|