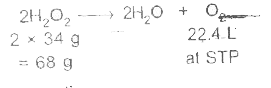Text Solution
Verified by Experts
Topper's Solved these Questions
HYDROGEN
AAKASH INSTITUTE ENGLISH|Exercise EXERCISE|10 VideosHYDROGEN
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (SECTION - A) (objective type question)|29 VideosHYDROGEN
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section-J)|5 VideosHYDROCARBONS
AAKASH INSTITUTE ENGLISH|Exercise Assignment(Section - C) (Previous Years Questions)|60 VideosM0ck test 26
AAKASH INSTITUTE ENGLISH|Exercise EXAMPLE|43 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-HYDROGEN-EXAMPLES
- Discuss the resemblance of hydrogen with halogens.
Text Solution
|
- Which isotope of hydrogen is radioactive in nature?
Text Solution
|
- Which isotope of hydrogen contains equal number of protons and neutron...
Text Solution
|
- Comment on the reaction of dihydrogen with fluorine.
Text Solution
|
- Explain the use of hydrogen in the formation of vegetable fats.
Text Solution
|
- Which class of covalent hydrides are considered as lewis acids?
Text Solution
|
- Association of molecules in water is due to:
Text Solution
|
- Describe the nature of ionic hydrides.
Text Solution
|
- Why do alcohol (a covalent compound) dissolves in water (ionic)?
Text Solution
|
- Which properties of hydrogen as responsible for moderation of the clim...
Text Solution
|
- Compare the density of ice and water.
Text Solution
|
- Does water gets oxidised in the process of photosynthesis?
Text Solution
|
- What type of water forms scum with soap?
Text Solution
|
- Write the reaction that takes place on adding lime to water containing...
Text Solution
|
- Explain with the help of reactions that how heavy water is used in the...
Text Solution
|
- What is calgon?
Text Solution
|
- Calculate the strength of 30 volume solution of hydrogen peroxide.
Text Solution
|
- What is the percentage strength of a solution of 100 volume H(2)O(2)?
Text Solution
|
- Why H(2)O(2) is kept away from dust?
Text Solution
|
- PbS(s)+4H(2)O(2)(aq)toPbSO(4)(s)+4H(2)O(l) In the above reaction, H(...
Text Solution
|