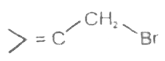
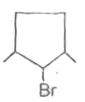
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HALOALKANES AND HALOARENES
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT SECTION -D|15 VideosHALOALKANES AND HALOARENES
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT SECTION - B|25 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
AAKASH INSTITUTE ENGLISH|Exercise Try Yourself|33 VideosHYDROCARBONS
AAKASH INSTITUTE ENGLISH|Exercise Assignment(Section - C) (Previous Years Questions)|60 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-HALOALKANES AND HALOARENES -ASSIGNMENT SECTION - C
- Which of the following undergoes nucleophilic substitution exclusively...
Text Solution
|
- The chirality of the compound
Text Solution
|
- C(5)H(11)Br+ NaCN rarr A overset(H(3)O^(+))(rarr) B + "NaOH" overset("...
Text Solution
|
- CD(2) = CH - CH(2) - Br is subjected to S(N)1 and S(N)2 reactions sepa...
Text Solution
|
- In Finkelstein reaction when acetone is replaced by water then
Text Solution
|
- Write brief notes on the following:(iii) Enzyme catalysis
Text Solution
|
- Which will undergo fastest S(N)2 substitution reaction when treated wi...
Text Solution
|
- Given reaction Y in the reaction is
Text Solution
|
- Which one of the following alkyl bromides undergoes most rapid solvoly...
Text Solution
|
- Monobrominaton of 2-methylbutane gives how many distinct isomers ?
Text Solution
|
- When CH(3)CH(2)CHCl(2) is treated with "NaNH"(2) the product formed is...
Text Solution
|
- Grignard reagent is prepared by the reaction between:
Text Solution
|
- The following reaction is described as
Text Solution
|
- 2-Bromopentane is heated with potassium ethoxide in ethanol. The majo...
Text Solution
|
- Which of the following compounds is not chiral ?
Text Solution
|
- An organic compound A(C(4)H(9)Cl) on the reaction with Na/diethly ethe...
Text Solution
|
- Reactivity order of halides for dehydrohalogenation is : -
Text Solution
|
- CH3CH2Cloverset(NaCN)to Xoverset(Ni//H2)to Yunderset("anhydride")overs...
Text Solution
|
- Which of the following is least reactive in a nucleophilic substitutio...
Text Solution
|
- Which of the following is not chiral:-
Text Solution
|