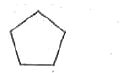
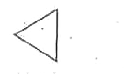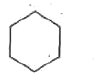A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HYDROCARBONS
AAKASH INSTITUTE ENGLISH|Exercise Assignment(Section - A) (Obejctive type question)|26 VideosHYDROCARBONS
AAKASH INSTITUTE ENGLISH|Exercise Assignment(Section - B) (Obejctive type question)|5 VideosHYDROCARBONS
AAKASH INSTITUTE ENGLISH|Exercise Try yourself|78 VideosHALOALKANES AND HALOARENES
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT SECTION -D|15 VideosHYDROGEN
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (SECTION - D) (Assertion-Reason Type question)|15 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-HYDROCARBONS-Exercise
- Which of the following halogens is the most reactive?
Text Solution
|
- Which of the following alkane upon dichlorination can give only two pr...
Text Solution
|
- Which of the following has maximum angle strain ?
Text Solution
|
- Total number of conformations possible in cyclohexane is
Text Solution
|
- Conformations arise due to rotation around
Text Solution
|
- Which of the following is the most stable cycloalkane ?
Text Solution
|
- Bond angle in chair form of cyclohexane is
Text Solution
|
- Most stable conformation of n-butane is :
Text Solution
|
- Torsion strain is the repulsive interaction between
Text Solution
|
- Which form (s) of cyclohexane is/are free from angle strain?
Text Solution
|
- The number of axial hydrogen atoms in chair form of cyclohexane is
Text Solution
|
- Consider the given reaction CH(3)-underset(CH(3))underset(|)overset(...
Text Solution
|
- Ethylene reacts with S(2)Cl(2) to give
Text Solution
|
- Actylene reacts with ammonical Cu(2)Cl(2) to give precipitate of
Text Solution
|
- Identify the product in the reaction HC-=CH overset(K(2)Cr(2)O(7)+H(...
Text Solution
|
- Which of the following compound gives CO(2) on reductive ozonolysis-
Text Solution
|
- The carbon-carbon bond length in benzene molecule is:
Text Solution
|
- The product formed in the reaction, Product is
Text Solution
|
- Which of the following is the most reactive towards nucleophilic subst...
Text Solution
|
- Which of the following is aromatic in nature ?
Text Solution
|