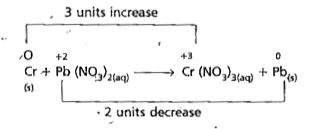Text Solution
Verified by Experts
Topper's Solved these Questions
STOICHIOMETRY
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise SHORT ANSWER QUESTIONS |88 VideosSTOICHIOMETRY
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise LONG ANSWER QUESTIONS |6 VideosSTOICHIOMETRY
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise Important Question |39 VideosSTATES OF MATTER GASES AND LIQUIDS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise LONG ANSWER QUESTIONS|22 VideosTHE P-BLOCK ELEMENTS GROUP-13
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise Long Answer Questions|3 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-STOICHIOMETRY -VERY SHORT Answer questions
- How many number of moles of glucose are present in 540 gms of glucose ...
Text Solution
|
- Calculate the weight of 0.1 mole of sodium carbonate.
Text Solution
|
- How many molecules of glucose are present in 5.23g of glucose (Molecul...
Text Solution
|
- Calculate the number of molecules persent in 1.12xx10^(-7) c.c. of a g...
Text Solution
|
- The empirical formula of a compound is CH(2)O. Its molecular weight is...
Text Solution
|
- Balance the following equation by the oxidation number method. Cr((s...
Text Solution
|
- What volume of H(2) at STP is required to reduce 0.795 g of CuO to giv...
Text Solution
|
- Calculate the volume of O(2) at STP required to completely burn 100 ml...
Text Solution
|
- Now a days it is thought that oxidation is simply decrease in electron...
Text Solution
|
- What is a redox concept? Give an example.
Text Solution
|
- Calculate the mass percent of the different elements present in sodium...
Text Solution
|
- What do you mean by significant figures?
Text Solution
|
- If the speed of light is 3.0xx10^(8)ms^(-1). Calculate the distance co...
Text Solution
|