Text Solution
Verified by Experts
Topper's Solved these Questions
STOICHIOMETRY
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise LONG ANSWER QUESTIONS |6 VideosSTOICHIOMETRY
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise Important Question |39 VideosSTOICHIOMETRY
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise VERY SHORT Answer questions |13 VideosSTATES OF MATTER GASES AND LIQUIDS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise LONG ANSWER QUESTIONS|22 VideosTHE P-BLOCK ELEMENTS GROUP-13
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise Long Answer Questions|3 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-STOICHIOMETRY -SHORT ANSWER QUESTIONS
- How much minimum volume of CO at STP is needed to react completely wit...
Text Solution
|
- Chemical analysis of a carbon compound gave the following percentage c...
Text Solution
|
- A carbon compound on analysis gave the following percentage compositio...
Text Solution
|
- Calculate the empirical formula of a compound having percentage compos...
Text Solution
|
- A carbon compound contains 12.8% Carbon, 2.1% Hydrogen, 85.1% Bromine....
Text Solution
|
- 0.188 g of an organic compound having an empirical formula CH(2)Br dis...
Text Solution
|
- Calculate the amount of 90% H(2)SO(4) required for the preparation of ...
Text Solution
|
- An astronaut receives the energy required in his body by the combustio...
Text Solution
|
- What volume of CO(2) is obtained at STP by heating 4 g of CaCO(3) ?
Text Solution
|
- When 50 gm of a sample of sulphur was burnt in air 4% of the sample wa...
Text Solution
|
- Calculate the volume of oxygen gas required at STP conditions for the ...
Text Solution
|
- Calculate the volume of H(2) liberated at 27^(@)C and 760 mm of Hg pre...
Text Solution
|
- Explain the role of redox reactions in titrimetre processes and galvan...
Text Solution
|
- Define and explain molar mass.
Text Solution
|
- What are disproportionate reactions? Give example.
Text Solution
|
- What is comproportionation reactions? Give example.
Text Solution
|
- Determine the empirical formula of an oxide of iron which has 69.9% ir...
Text Solution
|
- Calculate the mass of sodium acetate (CH(3)COONa) required to make 500...
Text Solution
|
- What is the concentration of sugar (C(12)H(22)O(11)) in mol L^(-1) if ...
Text Solution
|
- How many significant figures are present in the following ? i) 0.002...
Text Solution
|
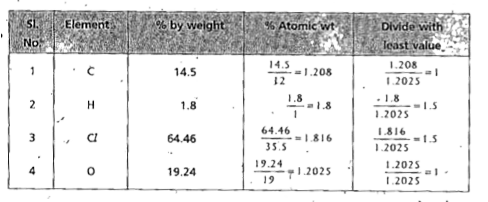 :. The formula of the compound = `C_(1)H_(1.5)Cl_(1.5)O_(1)`
:. The formula of the compound = `C_(1)H_(1.5)Cl_(1.5)O_(1)`