Text Solution
Verified by Experts
Topper's Solved these Questions
STOICHIOMETRY
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise LONG ANSWER QUESTIONS |6 VideosSTOICHIOMETRY
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise Important Question |39 VideosSTOICHIOMETRY
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise VERY SHORT Answer questions |13 VideosSTATES OF MATTER GASES AND LIQUIDS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise LONG ANSWER QUESTIONS|22 VideosTHE P-BLOCK ELEMENTS GROUP-13
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise Long Answer Questions|3 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-STOICHIOMETRY -SHORT ANSWER QUESTIONS
- Justify that the following reactions are redox reactions :
Text Solution
|
- Justify that the following reactions are redox reactions :
Text Solution
|
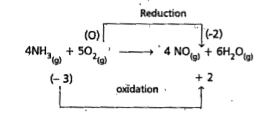
- Justify that the following reactions are redox reactions : 4 NH(3)...
Text Solution
|
- Fluorine reacts with ice and results in the change. H(2)O(s)+F(2)(g)...
Text Solution
|
- Calculate the oxidation number of sulphur, chromium and nitrogen ion H...
Text Solution
|
- Write the formulae for the following compounds. a) Mercury (II) chlo...
Text Solution
|
- Suggest a list of the substances where carbon exhibit oxidation states...
Text Solution
|
- While sulphue dioxide and hydrogen peroxide and act as oxidising as we...
Text Solution
|
- Consider the reactions a) 6CO(2)(g)+6H(2)O(I)toC(6)H(12)O(6)(aq)+6O(...
Text Solution
|
- The compound AgF(2) is unstable compound. However, if formed, the comp...
Text Solution
|
- Whenever a reaction between an oxidising agent and a reducing agent is...
Text Solution
|
- How do you count the following observations? a) Though alkaline pota...
Text Solution
|
- Identify the substance oxidised, reduced, oxidising agent and reducing...
Text Solution
|
- Identify the substance oxidised, reduced, oxidising agent and reducing...
Text Solution
|
- Identify the substance oxidised, reduced, oxidising agent and reducing...
Text Solution
|
- Identify the substance oxidised, reduced, oxidising agent and reducing...
Text Solution
|
- Identify the substance oxidised, reduced, oxidising agent and reducing...
Text Solution
|
- Consider the reactions 2S(2)O(3)^(2-)(aq)+I(2)(s)toS(4)O(6)^(2-)(aq)...
Text Solution
|
- Justify giving reactions that among halogens, fluorine is the best oxi...
Text Solution
|
- Why does the following reaction occur ? XeO(6)^(4-)(aq)+2F^(-)(aq)+6...
Text Solution
|