Text Solution
Verified by Experts
Topper's Solved these Questions
STOICHIOMETRY
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise Important Question |39 VideosSTOICHIOMETRY
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise SHORT ANSWER QUESTIONS |88 VideosSTATES OF MATTER GASES AND LIQUIDS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise LONG ANSWER QUESTIONS|22 VideosTHE P-BLOCK ELEMENTS GROUP-13
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise Long Answer Questions|3 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-STOICHIOMETRY -LONG ANSWER QUESTIONS
- Write the balanced ionic equation which represents the oxidation of io...
Text Solution
|
- Write the balanced ionic equation for the oxidation of sulphite ions t...
Text Solution
|
- Oxalic acid is oxidised by permanganate ion in acid medium of Mn^(2+) ...
Text Solution
|
- Phosphorus when heated with NaOH solution gives Phosphine (PH(3))andH(...
Text Solution
|
- Balance the following equation. Cr(OH)(3)+IO(3)^(-)overset(OH^(-))(t...
Text Solution
|
- Balance the following equation by the oxidation number method. MnO(4...
Text Solution
|
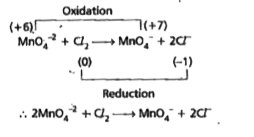 `:.` `2MnO_(4)^(-2)+Cl_(2) to MnO_(4)^(-)+2Cl^(-)`
`:.` `2MnO_(4)^(-2)+Cl_(2) to MnO_(4)^(-)+2Cl^(-)`