Text Solution
Verified by Experts
Topper's Solved these Questions
KTG & THERMODYNAMICS
RESONANCE ENGLISH|Exercise Solved problem|1 VideosKTG & THERMODYNAMICS
RESONANCE ENGLISH|Exercise Problem|3 VideosKTG & THERMODYNAMICS
RESONANCE ENGLISH|Exercise SUBJECTIVE QUESTIONS|27 VideosKINETIC THEORY OF GASES AND THERMODYNAMICS
RESONANCE ENGLISH|Exercise Exercise|64 VideosMAGNETIC FIELD AND FORCES
RESONANCE ENGLISH|Exercise Exercise|64 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-KTG & THERMODYNAMICS-Examples
- The cylinder shown in the figure has conducting walls and temperature ...
Text Solution
|
- A non conducting piston of mass m and area of cross section A is place...
Text Solution
|
- Find out the work done in the given graph. Also draw the corresponding...
Text Solution
|
- T-V curve of cyclic process is shown below, number of moles of the gas...
Text Solution
|
- P-T curve of a cyclic process is shown. Find out the works done by the...
Text Solution
|
- In figure, a cyclic process ABCA of 3 moles of an ideal gas is given. ...
Text Solution
|
- 1gm water at 100^(@)C is heated to convert into steam at 100^(@)C at 1...
Text Solution
|
- Two moles of a diatomic gas at 300 K are kept in a nonconducting conta...
Text Solution
|
- In figure, a sample of an ideal gas is taken through the cyclic proces...
Text Solution
|
- Two moles of nitrogen gas is kept in a cylinder of cross-section area ...
Text Solution
|
- An ideal gas intially has pressure P volume V and temperature T. Its i...
Text Solution
|
- A diatomic gas is heated at constant pressure. If 105J of heat is give...
Text Solution
|
- n moles of a diatomic gas has undergone a cyclic process ABC as shown ...
Text Solution
|
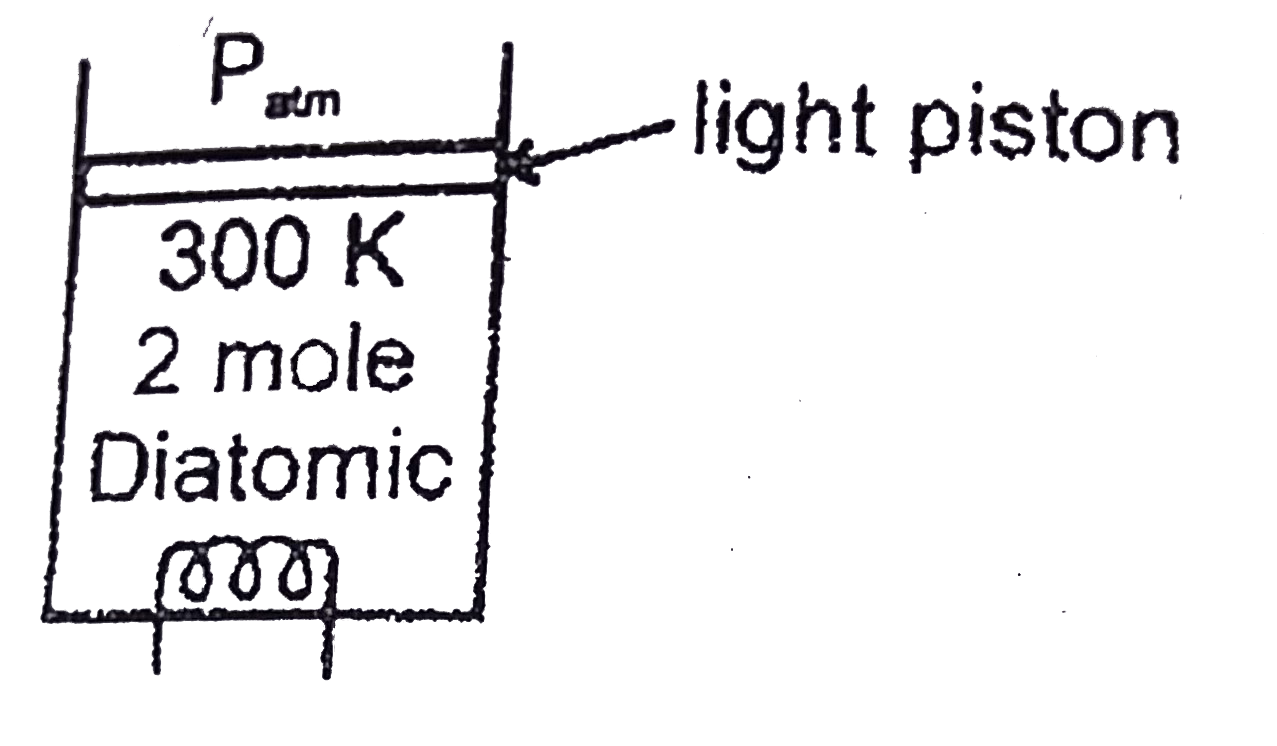
- Two moles of a diatomic gas at 300K are enclosed in a cylinder as show...
Text Solution
|
- P-V curve of a diatomic gas is shown in the Fig. Find the total heat g...
Text Solution
|
- Calculate the value of mechanical equivalent of heat from the followin...
Text Solution
|
- A container having slightly conducting walls contains air. The initial...
Text Solution
|
- A monoatomic gas is enclosed in a nonconducting cylinder having a pist...
Text Solution
|
- A cylindrical container having non-conducting walls is partitioned in ...
Text Solution
|
- A nonconducting cylinder having volume 2 V(0) is partitioned by a fixe...
Text Solution
|