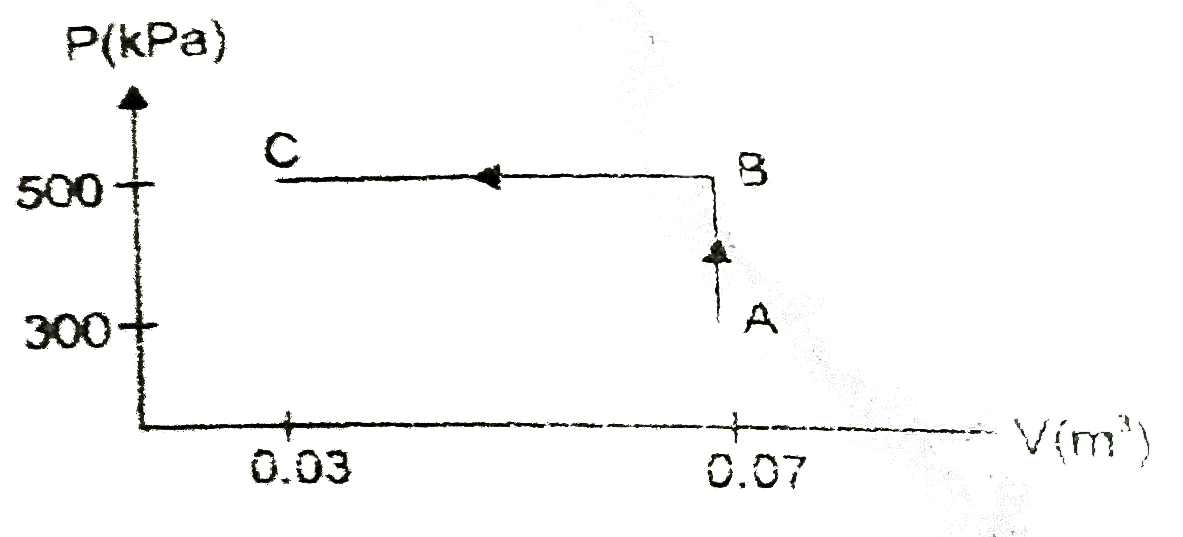Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
KTG & THERMODYNAMICS
RESONANCE ENGLISH|Exercise Exercise-2|1 VideosKTG & THERMODYNAMICS
RESONANCE ENGLISH|Exercise PART -I|15 VideosKTG & THERMODYNAMICS
RESONANCE ENGLISH|Exercise PART -II|17 VideosKINETIC THEORY OF GASES AND THERMODYNAMICS
RESONANCE ENGLISH|Exercise Exercise|64 VideosMAGNETIC FIELD AND FORCES
RESONANCE ENGLISH|Exercise Exercise|64 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-KTG & THERMODYNAMICS-SECTION
- Find the change in the internal energy of 2 kg of water as it heated f...
Text Solution
|
- In given figure, An ideal gas a gas is taken through a cyclic process ...
Text Solution
|
- An ideal gas is taken through the process ABC as shown in figure. If t...
Text Solution
|
- In given figure, one mole of an ideal gas (gamma = 7//5) is taken thro...
Text Solution
|
- 70 calories of heat required to raise the temperature of 2 moles of an...
Text Solution
|
- Internal energy of two moles of an ideal gas at a temperature of 127^(...
Text Solution
|
- Ideal monoatomic gas is taken through a process dQ = 2dU. Find the mol...
Text Solution
|
- Calculate the value of mechanical equivalent of heat from the followin...
Text Solution
|
- Find the change in internal energy of 2 moles of an ideal gas when its...
Text Solution
|
- When 100J of heat is given to an ideal gas it expands from 200cm^(3) t...
Text Solution
|
- The temperature of 5 mol of gas which was held at constant volume was ...
Text Solution
|
- For a gas gamma = 9//7. What is the number of degrees of freedom of th...
Text Solution
|
- If one mole of a monatomic gas (gamma=5/3) is mixed with one mole of a...
Text Solution
|
- The pressure and density of a diatomic gas (gamma=7//5) change adiabat...
Text Solution
|
- An ideal gas (gamma = (5)/(3)) is adiabatically compressed from 640 cm...
Text Solution
|
- In a adiabatic process pressure is increased by 2//3% if C(P)//C(V) = ...
Text Solution
|
- An ideal gas at pressure 4 xx 10^(5)Pa and temperature 400K occupies 1...
Text Solution
|
- In fig. the walls of the container and the piston are weakly conductin...
Text Solution
|
- When the state of a system changes from A to B adiabatically the work ...
Text Solution
|
- If Q amount of heat is given to a diatomic ideal gas in a process in w...
Text Solution
|