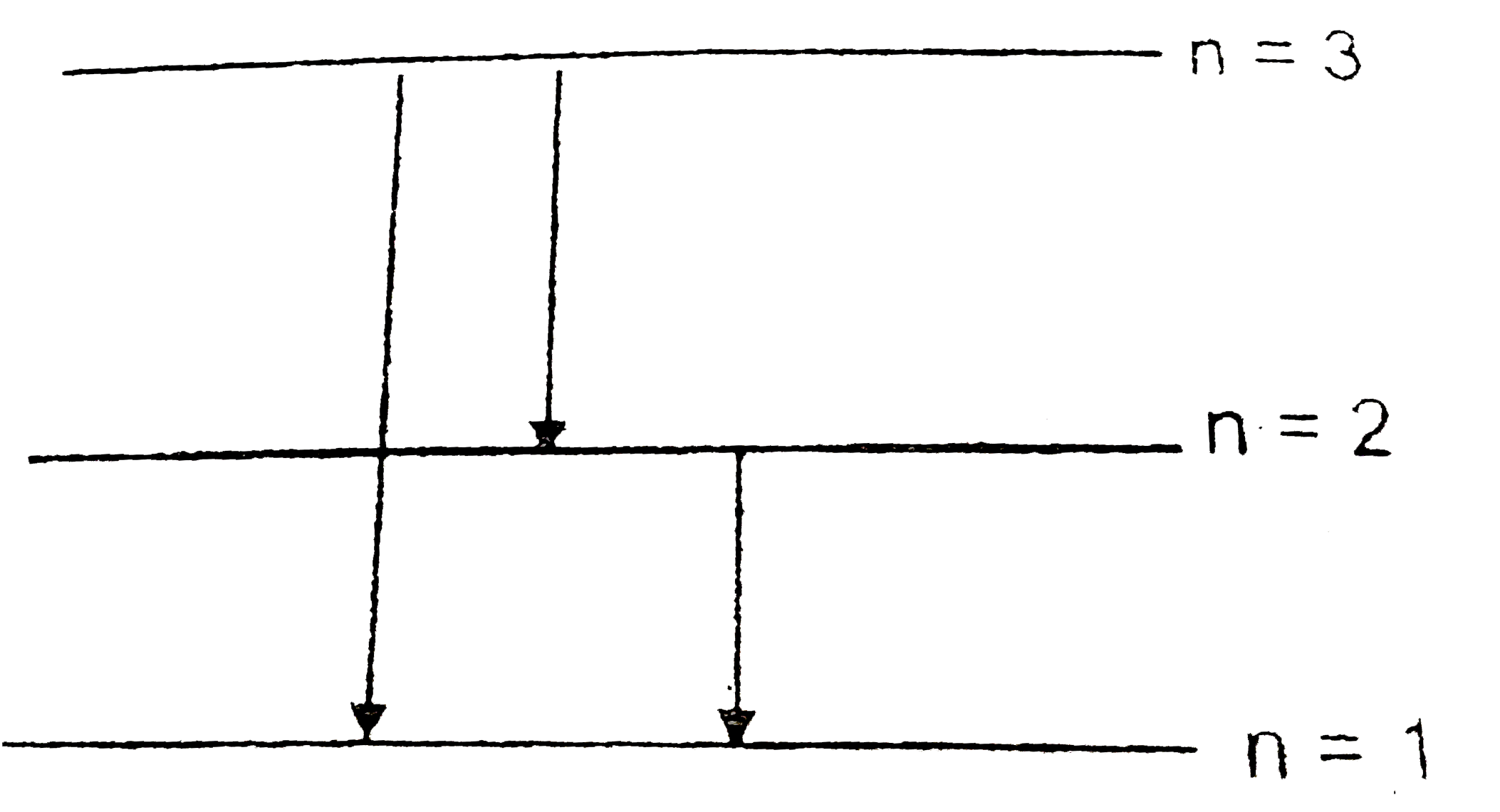
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
DAILY PRACTICE PROBLEM
RESONANCE ENGLISH|Exercise DPP No.21|20 VideosDAILY PRACTICE PROBLEM
RESONANCE ENGLISH|Exercise DPP No.22|9 VideosDAILY PRACTICE PROBLEM
RESONANCE ENGLISH|Exercise DPP No.19|20 VideosCURRENT ELECTRICITY
RESONANCE ENGLISH|Exercise High Level Problems (HIP)|19 VideosELECTRO MAGNETIC WAVES
RESONANCE ENGLISH|Exercise Exercise 3|27 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-DAILY PRACTICE PROBLEM-DPP No.20
- A metal surface is illuminated by light of two different wavelengths 2...
Text Solution
|
- The radius of the orbit of an electron in Hydrogen-like aton is 4.5 al...
Text Solution
|
- A cylinder rests in a supporting carriage as shown. The side AB of car...
Text Solution
|
- A painter is applying force himself to raise him and the box with an a...
Text Solution
|
- What is the minimum acceleration with which bar A (figure) should be s...
Text Solution
|
- Figure shows an ideal pulley block of mass m = 1 kg. resting on a roug...
Text Solution
|
- A meter stick AB of length 1 meter rests on a frictionless floor in ho...
Text Solution
|
- A meter stick AB of length 1 meter rests on a frictionless floor in ho...
Text Solution
|
- A meter stick AB of length 1 meter rests on a frictionless floor in ho...
Text Solution
|