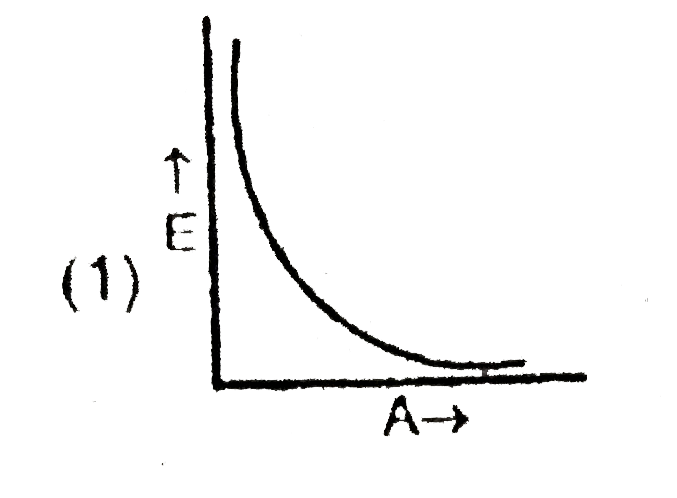
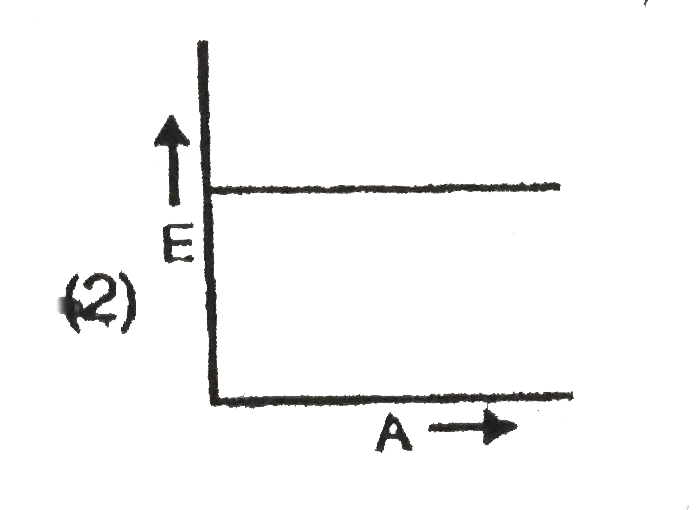
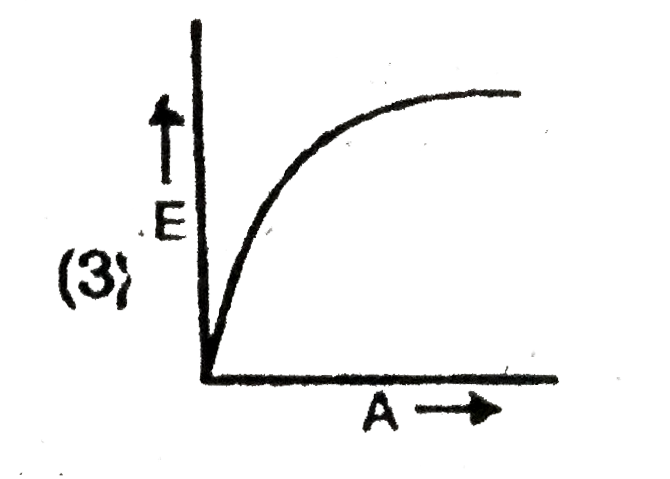
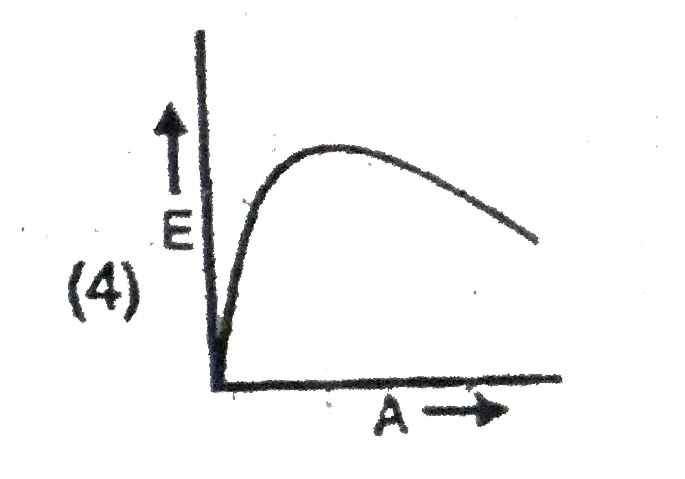
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-NUCLEAR PHYSICS-Exercise
- Slow neutron are sometimes refer to as thermal neutrons because
Text Solution
|
- Which of the following is correct about nuclear forces?
Text Solution
|
- The graph between the binding energy per nucleon (E) and atomic mass n...
Text Solution
|
- The probability of a radioactive atoms to survive 5 times longer than ...
Text Solution
|
- After emission of an alpha-particle by a radiative element .(84)X^(212...
Text Solution
|
- The SI unit of activity is-
Text Solution
|
- The specific activity of radius is nearly-
Text Solution
|
- After a time equal to four half lives, the amount of radioactive mater...
Text Solution
|
- The mean of life of a radioactive sample is 100 years. Then after 100 ...
Text Solution
|
- The count rate of 10 g of radioactive material was measured at differe...
Text Solution
|
- How many atoms decay in one mean life time of a radioactive sample-
Text Solution
|
- The half life of radioactive substance is T. Then the fraction of the ...
Text Solution
|
- The half-life of a radioactive substance is 3h and its activity is 1mu...
Text Solution
|
- The half -life of cobalt-60 is 5.25 years. How long after a new sample...
Text Solution
|
- A radioactive element ThA( .(84)Po^(216)) can undergo alpha and beta a...
Text Solution
|
- A radioactive nucleus undergoes a series of decay according to the sch...
Text Solution
|
- Compare the ionising power of alpha, beta and gamma radiations.
Text Solution
|
- In nuclear power station energy of uranium is used for producing-
Text Solution
|
- The order of magnitude of the density of nuclear matter is=
Text Solution
|
- The Process by which a heavy nucleus splits into light nuclei is known...
Text Solution
|