Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-NUCLEAR PHYSICS-Exercise -3 Part-III CBSE PROBLEMS (LAST 10 YEARS)
- Draw the graph showing thervariation of binding energy per nucleon wit...
Text Solution
|
- Name the reaction which takes place when a slow neutron beam strikes ....
Text Solution
|
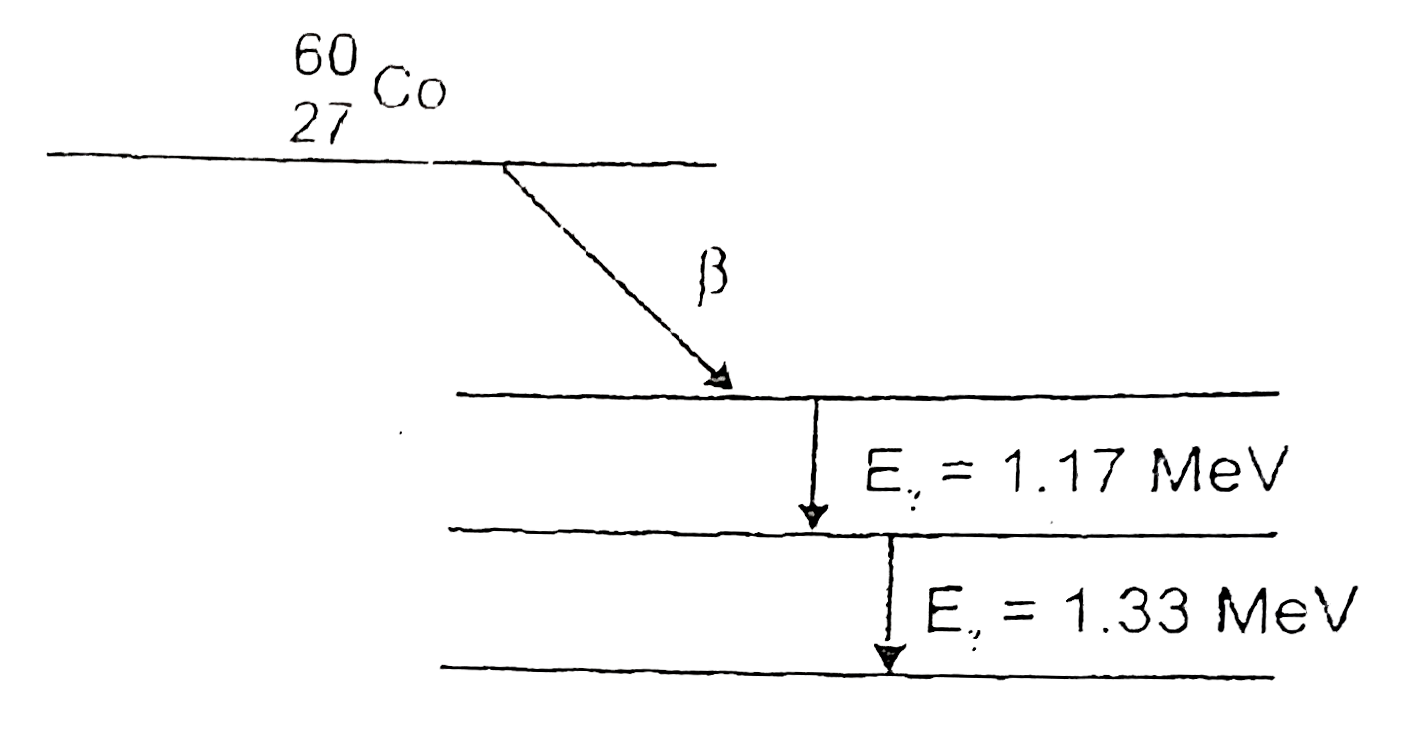
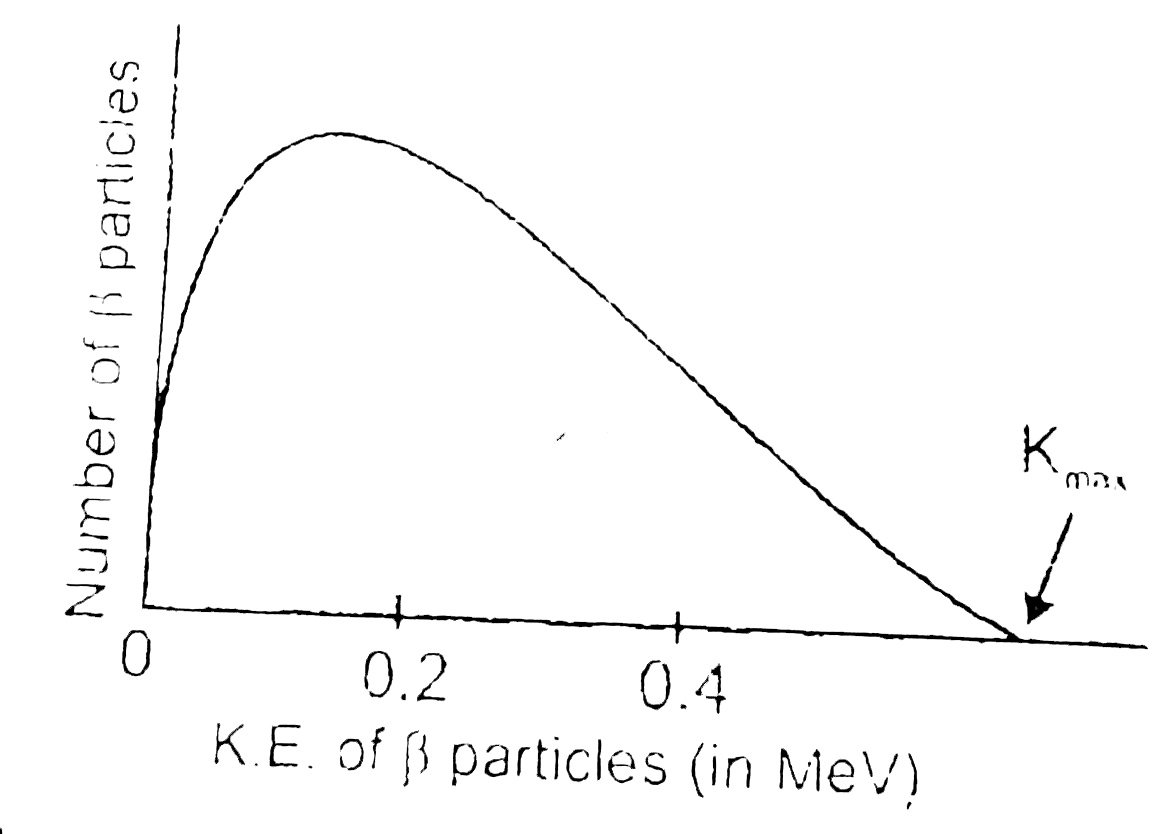
- (a) Draw the energy level diagram showing the emission of beta-particl...
Text Solution
|
- A radioactive sample contains 2.2 mg of pure .(6)^(11)C which has a ha...
Text Solution
|
- Define decay constant
Text Solution
|
- Define the term half-life period and decay constant of a radioactive s...
Text Solution
|
- Define 'activity' of a radioactive material and write its S.I.unit. ...
Text Solution
|
- Characteristic of Angiosperms which distinguish them from gymnosperms
Text Solution
|
- State and explain the laws of radioactive disintergration. Hence defin...
Text Solution
|
- The value of binding energy per nucleon of .(20)^(40)Ca nucleus is Giv...
Text Solution
|
- Calculate the energy released in MeV in the following nuclear reaction...
Text Solution
|
- Calculate the energy released in MeV in the following nuclear reaction...
Text Solution
|
- A neutron is absorbed by a .(3)^(6)Li nucleus with the subsequent emis...
Text Solution
|
- What do you understand by isotopes, isobars and isotones? Explain with...
Text Solution
|
- The nucleus ""(10)^(23) Ne decays by beta– emission. Write down the ...
Text Solution
|
- Two nuclei have mass numbers in the ratio 1 : 2. What is the ratio of ...
Text Solution
|
- A radioactive nucleus undergoes a series of deacy according to the sch...
Text Solution
|
- Name and define, the SI unit for the 'activity' of a given sample of r...
Text Solution
|
- Draw a plot of potential energy of a pair of nucleons as a function of...
Text Solution
|
- (a) Write symbolically the beta-decay process of ""(15)^(32)P. (b) ...
Text Solution
|