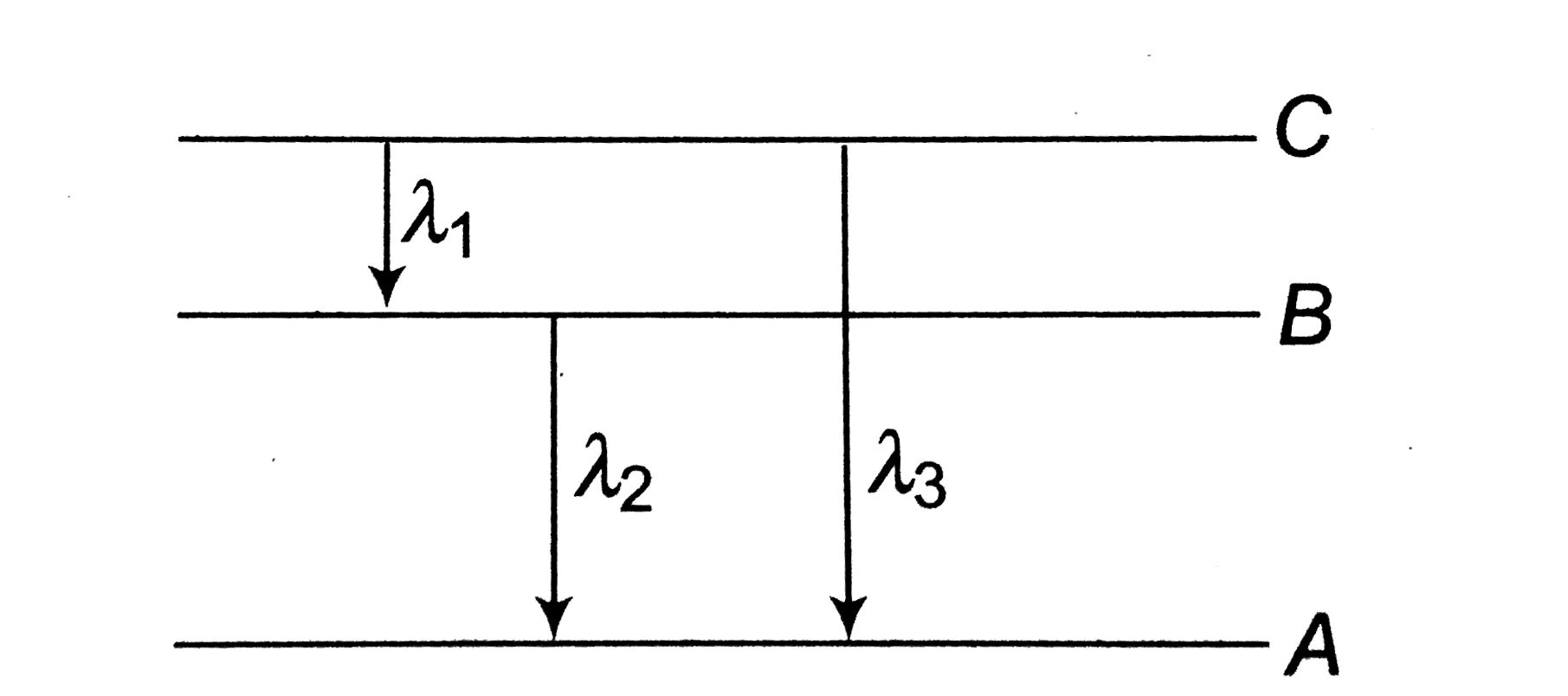
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC PHYSICS
RESONANCE ENGLISH|Exercise Exercise (2) Only one option correct type|30 VideosATOMIC PHYSICS
RESONANCE ENGLISH|Exercise Exercise-2 part-II Single and double value integer type|12 VideosATOMIC PHYSICS
RESONANCE ENGLISH|Exercise Exercise 1 Part-1 subjective questions|35 VideosALTERNATING CURRENT
RESONANCE ENGLISH|Exercise HIGH LEVEL PROBLEMS|11 VideosCAPACITANCE
RESONANCE ENGLISH|Exercise High Level Problems|16 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-ATOMIC PHYSICS-Exercise ( Part II : Only one one correct type)
- If a(0) is the Bohr radius, the radius of then n=2 electronic orbit in...
Text Solution
|
- Ionisation energy of a hydrogen-like ion A is greater than that of ano...
Text Solution
|
- Which energy state of doubly ionized lithium Li^(++) has the same ener...
Text Solution
|
- In Bohr's model of hydrogen atom a(0) is the radius of the ground stat...
Text Solution
|
- If an orbital electron of the hydrogen atom jumps from the ground stat...
Text Solution
|
- In the Bohr model of the hydrogen atom, the ratio of the kinetic energ...
Text Solution
|
- The innermost orbit of the hydrogen atom has a diameter of 1.06Å what ...
Text Solution
|
- Three photons coming from excited atomic-hydrogen sample are picked up...
Text Solution
|
- The transition from the state n = 4 " to " n = 3 in a hydrogen like at...
Text Solution
|
- Ionization potential of hydrogen atom is 13.6eV . Hydrogen atoms in th...
Text Solution
|
- Energy levels A, B, C of a certain atom corresponding to increasing va...
Text Solution
|
- For the first member of Balmer series of hydrogen spectrum, the wavele...
Text Solution
|
- The frequency of the first line in Lyman series in the hydrogen spect...
Text Solution
|
- An electron of kinetic energy K collides elastically with a stationary...
Text Solution
|
- Consider a photon of continuous X-ray coming from a Coolidge tube. Its...
Text Solution
|
- If the current in the circuit for heating the filament is increased, t...
Text Solution
|
- The characterstics X-ray spectrum is emitted due to transition of
Text Solution
|
- When an ultraviolet light is incident on a photocell , its stopping po...
Text Solution
|
- In Davosson-Germer experiment, the filament emits
Text Solution
|
- In the Davisson and Germer experiment, the velocity of electrons emitt...
Text Solution
|