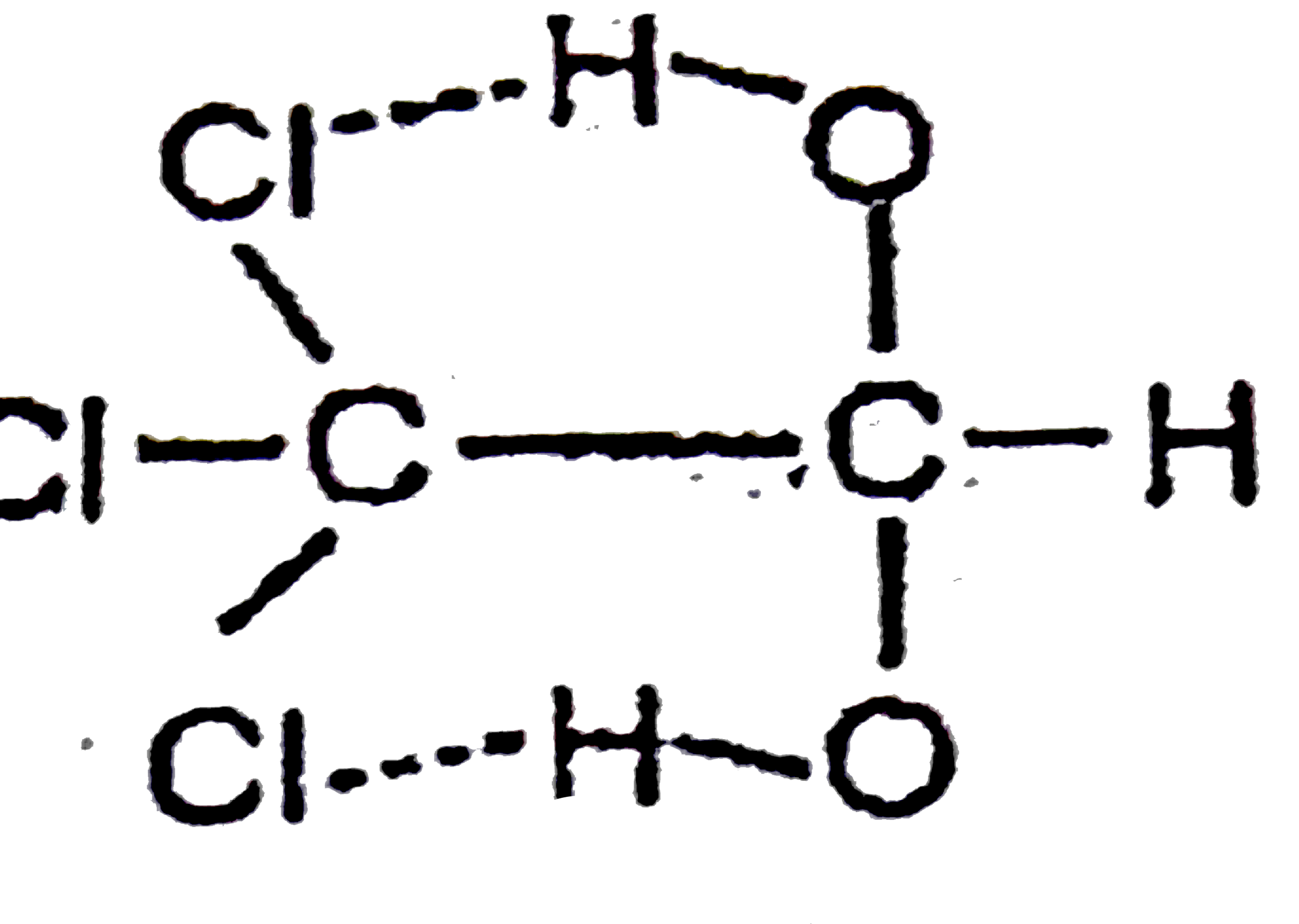
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC STRUCTURE
RESONANCE ENGLISH|Exercise Inorganic chemistry (Chemistry Bonding)|38 VideosATOMIC STRUCTURE
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Fundamental Concept )|16 VideosAROMATIC COMPOUNDS
RESONANCE ENGLISH|Exercise PART-II : JEE (MAIN)|1 VideosCHEMICAL BONDING
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Fundamental Concept )|6 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-ATOMIC STRUCTURE-ORGANIC CHEMISTRY(Fundamental Concept )
- A compound of vanadium has a magneitc moment (mu) of 1.73 BM. If the v...
Text Solution
|
- The magnetic moment of .25Mn in ionic state is sqrt(15)B.M, then Mn is...
Text Solution
|
- The spin only magnetic of Cr^(3+) in aqueous solution would be :
Text Solution
|
- psi^(2)=0 represent
Text Solution
|
- Observe the following statements regarding isotones : a. .^(39)K and...
Text Solution
|
- If n(1) and n(2) are the boundary value principal quantum numbers of a...
Text Solution
|
- What is the potential energy of an electron present in N- shell of th...
Text Solution
|
- In which of the following transition, the wavelength will be minimum ...
Text Solution
|
- The ratio of kinetic energy and potential energy of an electron in a B...
Text Solution
|
- Total Number of unpaired electrons in d- orbitals of an atom element o...
Text Solution
|
- Photons of minimum energy 496k,J. mol^(-1) are needed to an atoms. Cal...
Text Solution
|
- According to Bohr's model, if the kinetic energy of an electron in 2^(...
Text Solution
|
- Last line of Lyman series for H- atom has wavelength lambda(1) A,2^(nd...
Text Solution
|
- Which electronic level would allow the hydrogen atom to absorb a photo...
Text Solution
|
- Number of nomal planes ( planes of zero electron densit ) in the d(xy)...
Text Solution
|
- The uncertainty in position and velocity of the particle are 0.2mm and...
Text Solution
|
- A particle initially at rest having charge q coulomb, & mass m kg i...
Text Solution
|