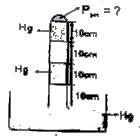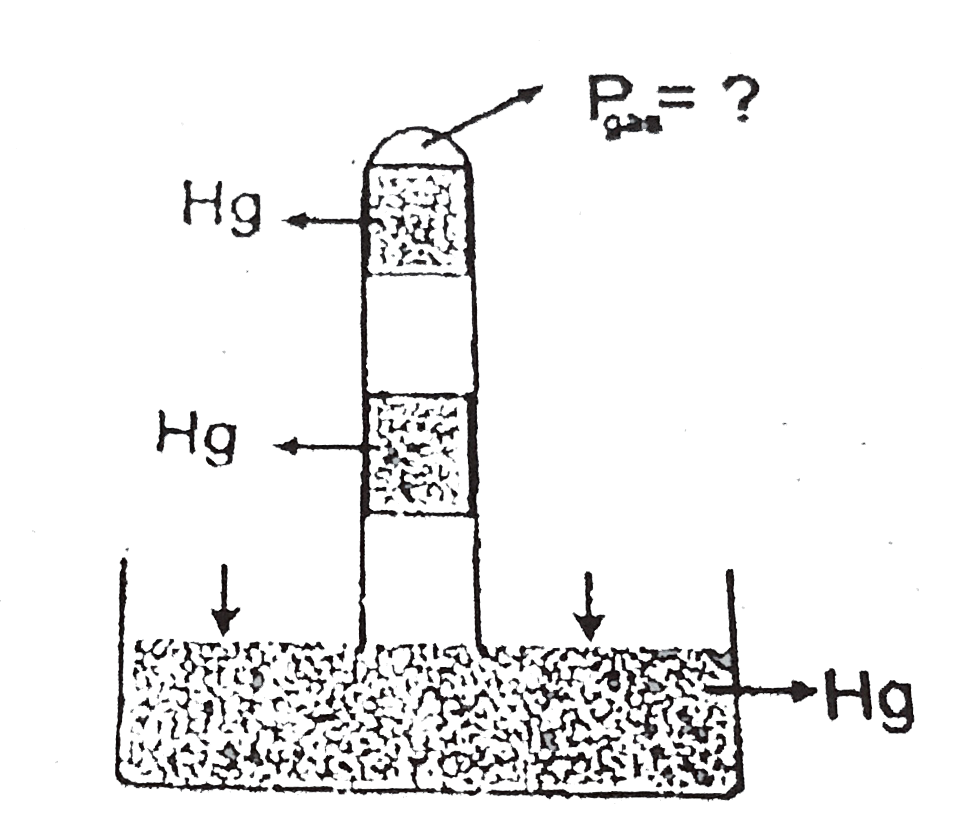A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GASEOUS STATE
RESONANCE ENGLISH|Exercise INORGANIC CHEMISTRY (COORDINATION COMPOUNDS)|36 VideosGASEOUS STATE
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Hydrocarbon)|18 VideosD & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS
RESONANCE ENGLISH|Exercise Match the column|1 VideosGENERAL ORGANIC CHEMISTRY II
RESONANCE ENGLISH|Exercise Part-III: Section-5: Matching List Type|1 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-GASEOUS STATE-ORGANIC CHEMISTRY(Hydrocarbon)
- In the above figure mercury columns of 10 cms each are trapped between...
Text Solution
|
- A certain quantity of a gas occupied 100ml when collected over water a...
Text Solution
|
- The virial equation for 1mole of a real gas is written as : PV=RT ...
Text Solution
|
- Infinite number of flask are connected to one another as shown above. ...
Text Solution
|
- At point P and Q , the real gas deviation with respect to ideal gas is...
Text Solution
|
- A real gas most closely approaches the behaviour of an ideal gas at:
Text Solution
|
- If the number of molecules of SO(2) (atomic weight=64) effusing throug...
Text Solution
|
- A gaseous mixture contains three gases A,B and C with a total number o...
Text Solution
|
- 1 mol of a gaseous aliphatic compound CnH(3n)Om is completely burnt in...
Text Solution
|
- At what temperature will the molar kinetic energy of 0.3 mol of He be ...
Text Solution
|
- The volume of a gas increases by a factor of 2 while the pressure dec...
Text Solution
|
- Which has maximum internal energy at 290 K?
Text Solution
|
- There are 6.02 xx 10^(22) molecules each of N(2),O(2) and H(2) which a...
Text Solution
|
- At 27^(@)C, a ges is compressed to half of its volume . To what temper...
Text Solution
|
- A gas in an open container is heated from 27^(@)C" to "127^(@)C. The ...
Text Solution
|
- A V dm^(3) flask contains gas A and another flask of 2V dm^(3) contain...
Text Solution
|
- At low pressure, vander waal’s equation is reduced to [P + (a)/(V^(2))...
Text Solution
|
- 300 ml of a gas at 27^(@)C is cooled to -3^(@)C at constant pressure, ...
Text Solution
|
- In the ideal gas equation, the gas constant R has the dimension of -
Text Solution
|