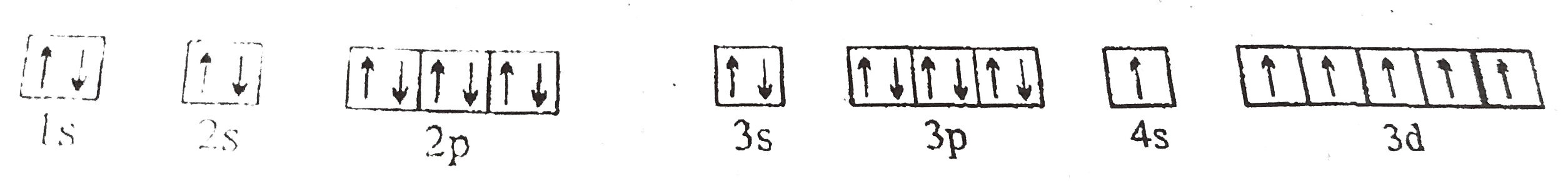
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING
RESONANCE ENGLISH|Exercise Inorganic chemistry (Chemistry Bonding)|49 VideosCHEMICAL BONDING
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Fundamental Concept )|6 VideosATOMIC STRUCTURE
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Fundamental Concept )|16 VideosCHEMICAL EQUILIBRIUM
RESONANCE ENGLISH|Exercise Advanced Level Problems (Part-3)(Stage-5)|2 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-CHEMICAL BONDING-ORGANIC CHEMISTRY(Fundamental Concept )
- Two types of carbon- carbon covalent bond lengths are present in
Text Solution
|
- The correct order of dipole moment is .
Text Solution
|
- Which of the following is not correct ?
Text Solution
|
- Correct order of bond length is:
Text Solution
|
- Which of the following is paramagnetic ?
Text Solution
|
- Gaseous SO(3) molecule
Text Solution
|
- Among the following, the pair in which the two species are not iso-str...
Text Solution
|