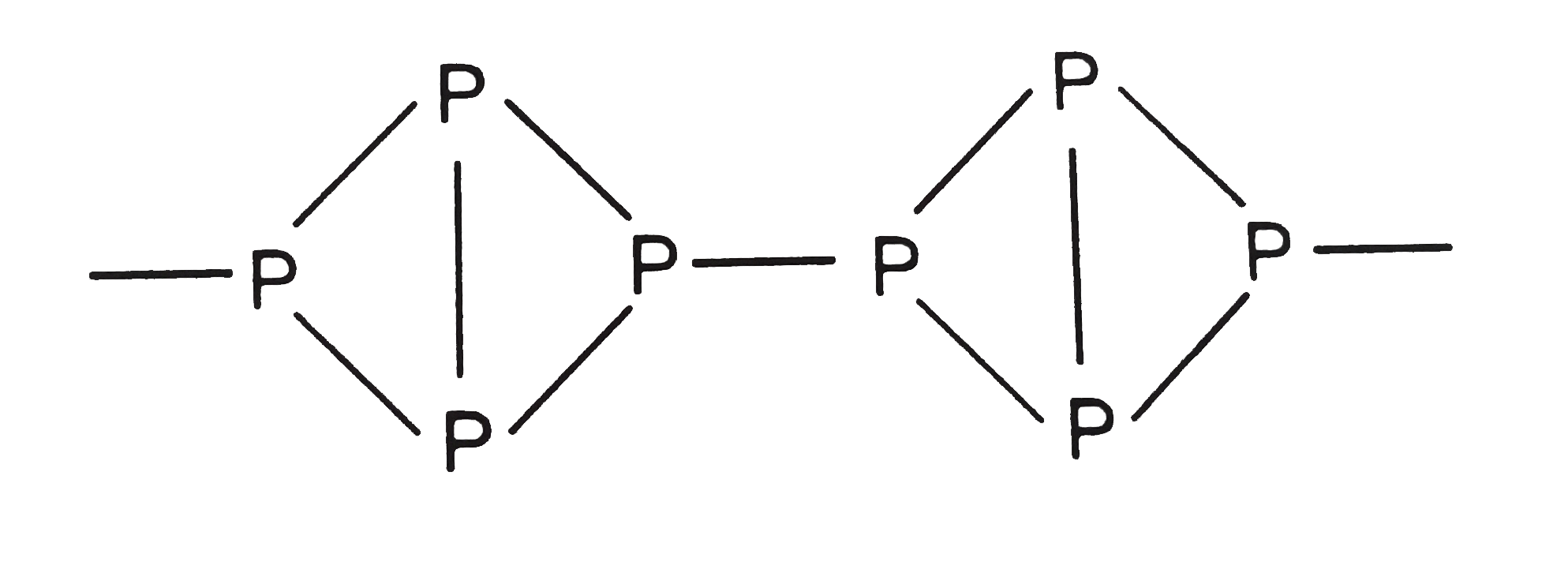
Text Solution
Verified by Experts
Topper's Solved these Questions
P BLOCK ELEMENTS
RESONANCE ENGLISH|Exercise B.L.E|49 VideosP BLOCK ELEMENTS
RESONANCE ENGLISH|Exercise Exercise 1 part 1 subjective ques|30 VideosP BLOCK ELEMENTS
RESONANCE ENGLISH|Exercise INORGANIC CHEMISTRY(P-Block Elements)|35 VideosNUCLEAR CHEMISTRY
RESONANCE ENGLISH|Exercise STAGE-II|1 VideosP-BLOCK ELEMENT (BORON AND CARBON FAMILY)
RESONANCE ENGLISH|Exercise PART - III : OLYMPIAD PROBLEMS (PREVIOUS YEARS) STAGE - V (INTERNATIONAL CHEMISTRY OLYMPIAD (IChO)) Problem 3|8 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-P BLOCK ELEMENTS-Example
- NH(3) + O(2) overset("Pt")underset(Delta)(to) A + H(2) O A + O(2) to...
Text Solution
|
- Why NH(3) gas cannot be dried by passing over P(2)O(5),CaCl(2) and H(2...
Text Solution
|
- Explain the high reactivity of white phosphorus as compared to red pho...
Text Solution
|
- What happens ? (a)When phosphine is heated at 150^(@)C. (b)When ph...
Text Solution
|
- P(4)+NaOH underset("warm")to Products. Write the products
Text Solution
|
- Identify the group 16 (VI A) element that fits each of the following d...
Text Solution
|
- Give the names and formulae of the compounds in which sulphur exhibits...
Text Solution
|
- O(3) is a powerful oxidising agent. Write equation to represent oxidat...
Text Solution
|
- Give the important applications of O(3)
Text Solution
|
- Black (A)+H(2)SO(4)to (B)"gas"+( C) (B)+(CH(3)COO)(2) Pb to (D) blac...
Text Solution
|
- SO(2) and Cl(2) both are used as bleaching agent. What factors cause b...
Text Solution
|
- In an atom with atomic no. 39 and mass no. 89,the number of electron i...
Text Solution
|
- In an atom with atomic no. 42 and mass no. 95,the number of electron i...
Text Solution
|
- Bond energies can be obtained by using the following relation: DeltaH ...
Text Solution
|
- In a reaction, DeltaH and DeltaS both are more than zero. In which of ...
Text Solution
|
- Br(2)+underset(HOT)OH^(o+)rarr(A)+(B) (A)+(B)+H^(o+)rarr Br(2) (A)...
Text Solution
|
- (a)When HCI reacts with finely powdered iron , it forms ferrous chlori...
Text Solution
|
- A 1.24 M aqueous solution of KI has a density of 1.15 gcm^(−3). What i...
Text Solution
|
- Identify A , B and C in the following
Text Solution
|
- Which of the following is a true nut?
Text Solution
|