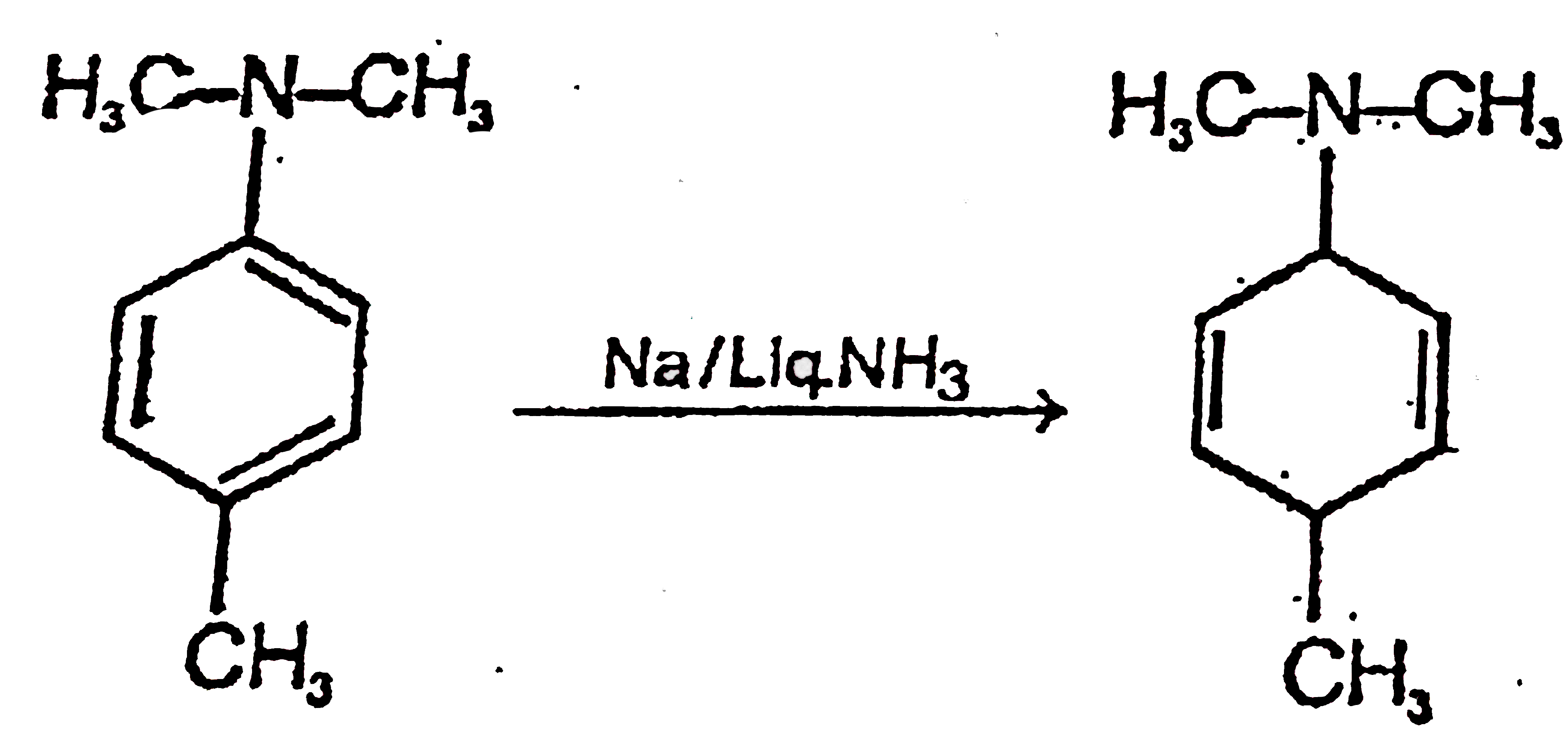A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-TEST PAPERS-PT-06
- Mnomer of [-underset(CH(3))underset(|)overset(CH(3))overset(|)C-CH(2)-...
Text Solution
|
- Meso-tartaric acid and d-tartaric acid are
Text Solution
|
- In which option products is not correct.
Text Solution
|
- Which compounds is used to prepare 1^(@) alcohol from Grignard reagen...
Text Solution
|
- formed product 'X' is used as:
Text Solution
|
- Which of the following acid remains unaffected on heating ?
Text Solution
|
- The monomer of the polymer
Text Solution
|
- How many grams of glucose be dissolved to make one litre solution of 1...
Text Solution
|
- A ball of mass 1 kg is released from position A inside a wedge with a ...
Text Solution
|
- An S(N^2) reaction occurs through the formation of a
Text Solution
|
- Which one of the following hyxachlorocyclohexane is least reactive and...
Text Solution
|
- Which of the following will not undergo aldol condensation?
Text Solution
|
- The compound formed when malonic acid is heated with urea, is :
Text Solution
|
- [X]overset(H(2)OSO(2))rarrunderset((2)H(3)O^(+))overset((1)KOH//Delta)...
Text Solution
|
- Identify the principal product of the following reaction ,
Text Solution
|
- The product Y is :
Text Solution
|
- Identify the final major product for the following reaction ? Anlin...
Text Solution
|
- Which statement is correct
Text Solution
|
- on ozonolysis gives :
Text Solution
|
- underset((0-5^(@)C))overset(NaNO(2)//HCl)rarr A overset(CuCN)rarrB ove...
Text Solution
|