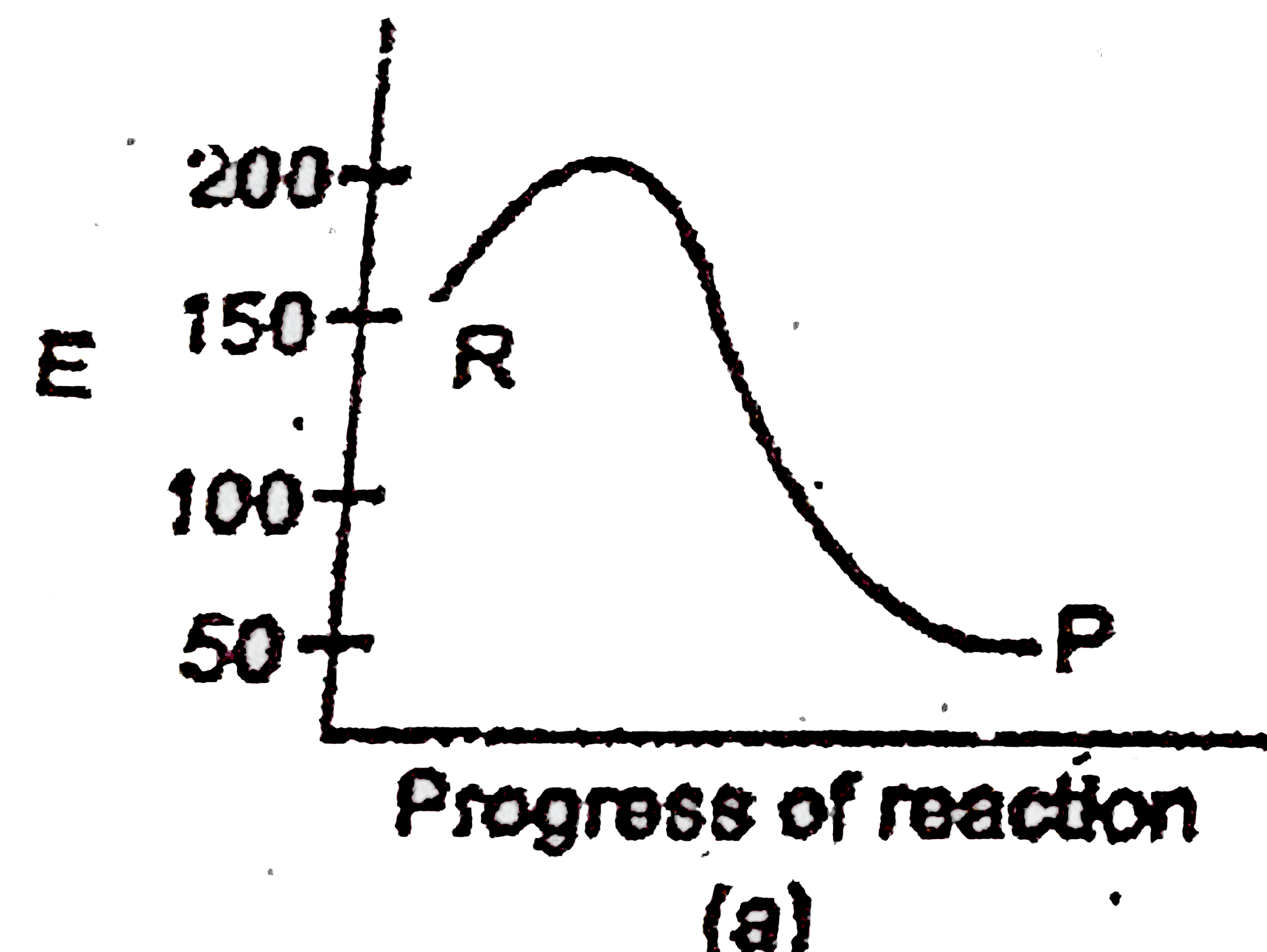
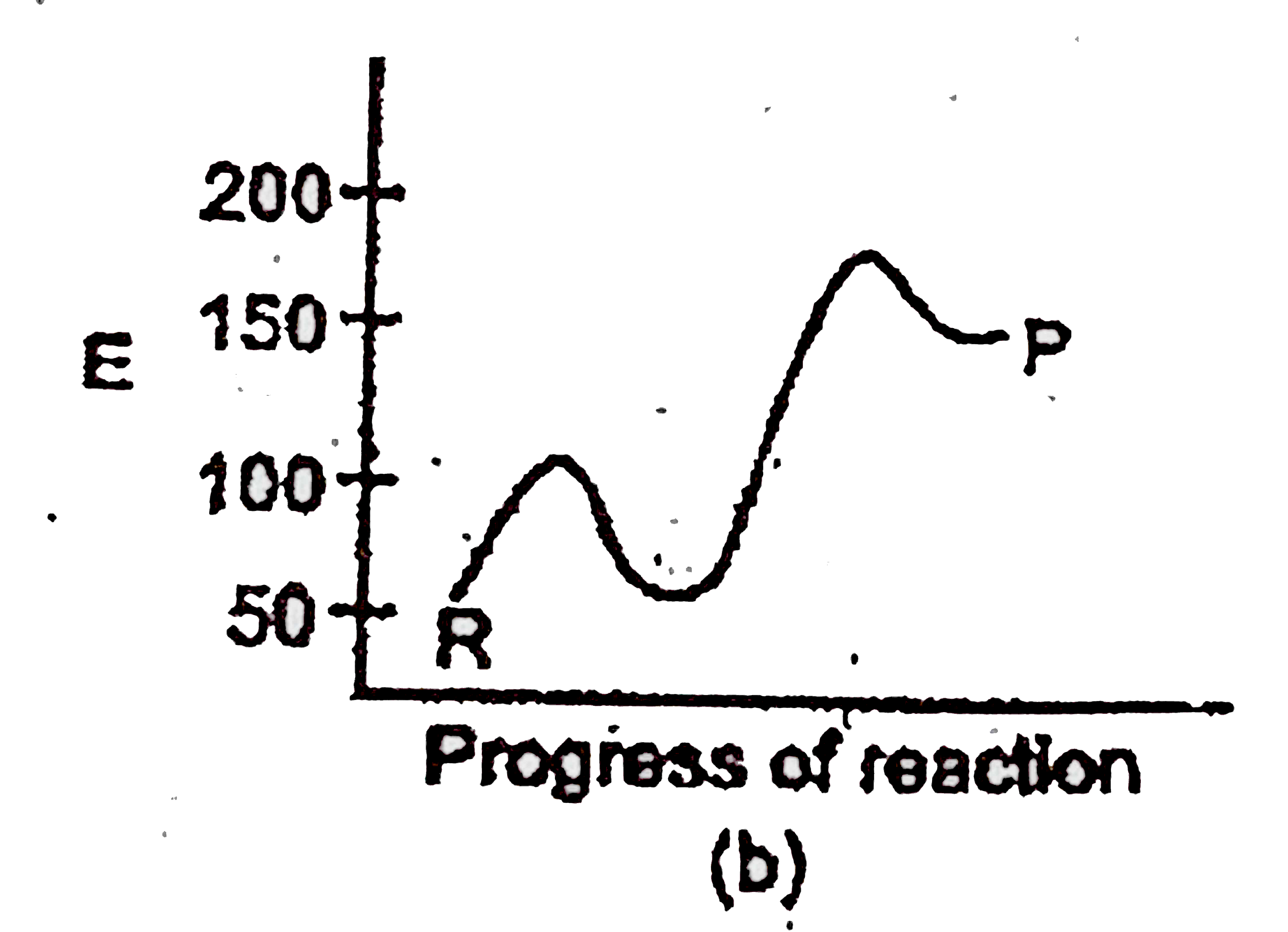
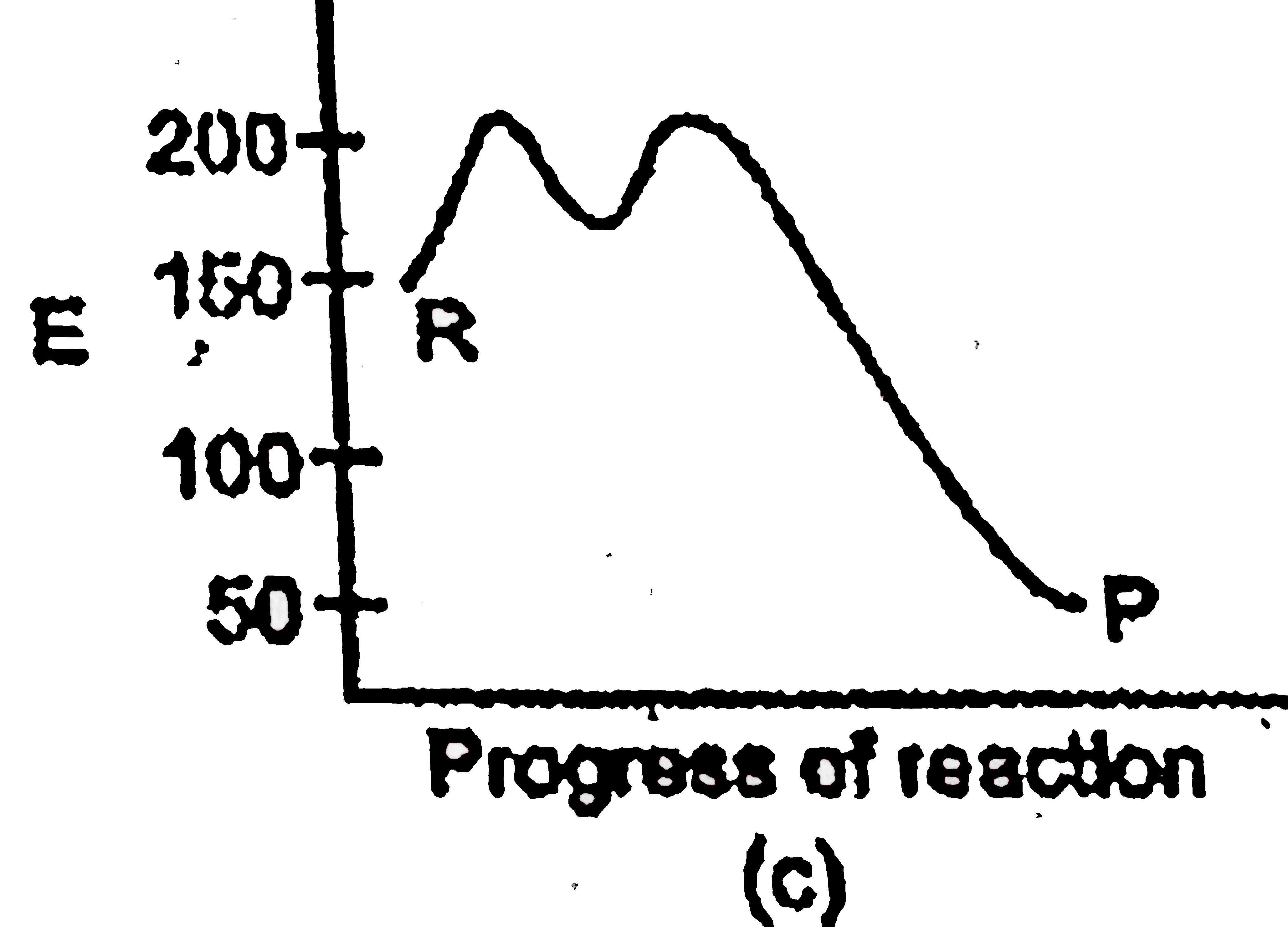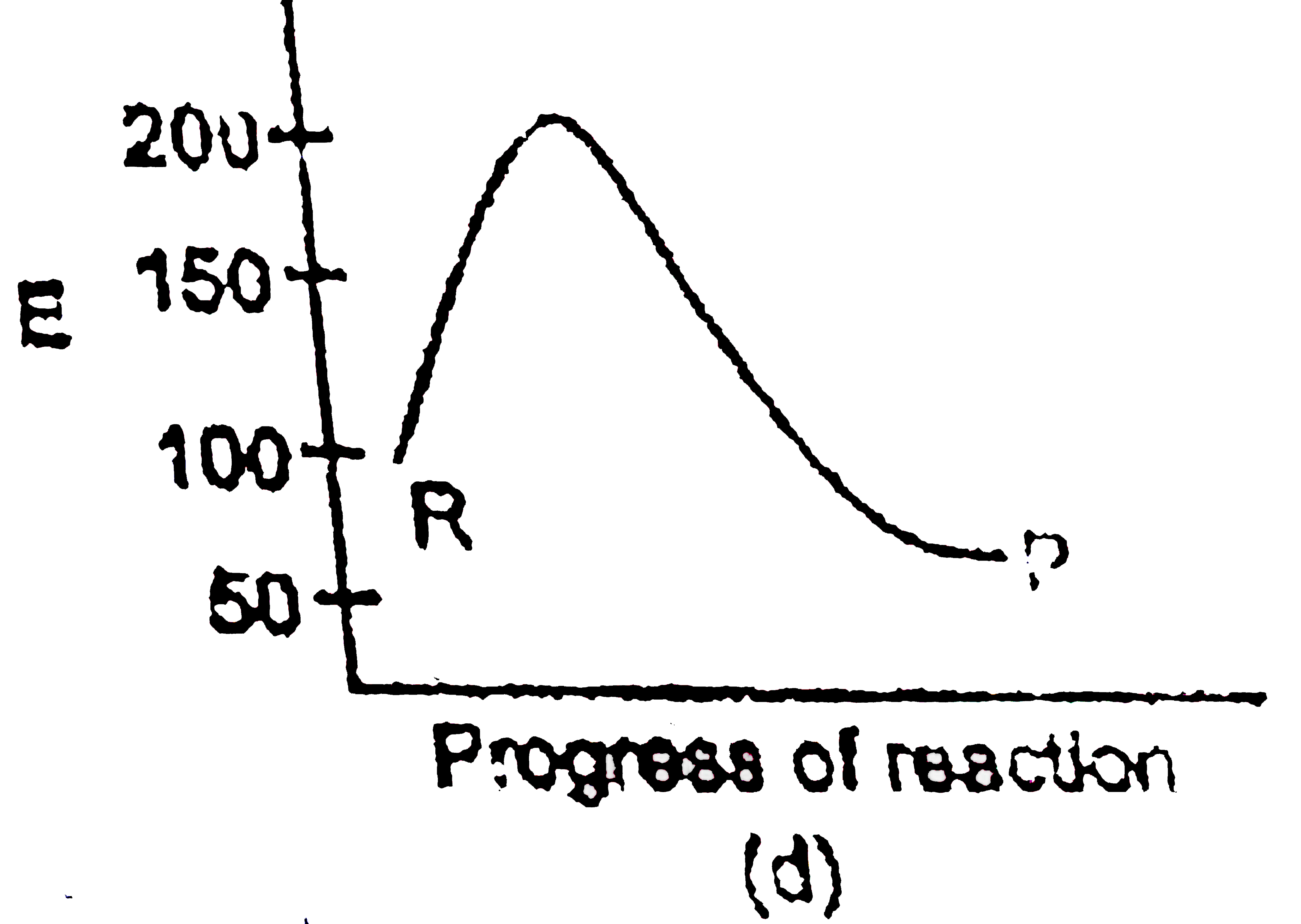A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-TEST PAPERS-FST-3
- Ultraviolet light of 6.2eV falls on Aluminium surface (work function =...
Text Solution
|
- When MnO(2) fused with KOH, a coloured compound is formed . The produc...
Text Solution
|
- Ultraviolet light of 6.2eV falls on Aluminium surface (work function =...
Text Solution
|
- Among the following statements the incorrect one is:
Text Solution
|
- Depression in f.p. of an aqueous urea solutio is 1.86^(@) .(K(f)=1.86^...
Text Solution
|
- Ultraviolet light of 6.2eV falls on Aluminium surface (work function =...
Text Solution
|
- An exothermic chemical reaction proceeds by two stages. Reactions ov...
Text Solution
|
- A metal X on heating in nitrogen gas gives Y,Y on treatment with H(2)O...
Text Solution
|
- In FCC lattice of NaCl structure, if the diameter of Na^(+) is x, and ...
Text Solution
|
- The kinetic energy of an electron accelerated from rest through a pote...
Text Solution
|
- A racemic mixture of 2- Chloropropanoic acid is treated with excess of...
Text Solution
|
- Which of the following compound is most reactive in the nucleophilic a...
Text Solution
|
- What is the ends product of following sequence of reaction
Text Solution
|
- Which of the following polymer matched with all the given characterist...
Text Solution
|
- Which of the following pair is isodiaphers?
Text Solution
|
- Which statement is incorrect?
Text Solution
|
- The following reaction is known as : Carbonyl compound underset(50%"...
Text Solution
|
- Identify Z in the following reaction sequence. CH(3)Iunderset("Ether...
Text Solution
|
- Ph-underset(Me)underset(|)overset(OH)overset(|)(C)-underset(I)underset...
Text Solution
|
- Which of the following reagent can be used for above conversion ?
Text Solution
|