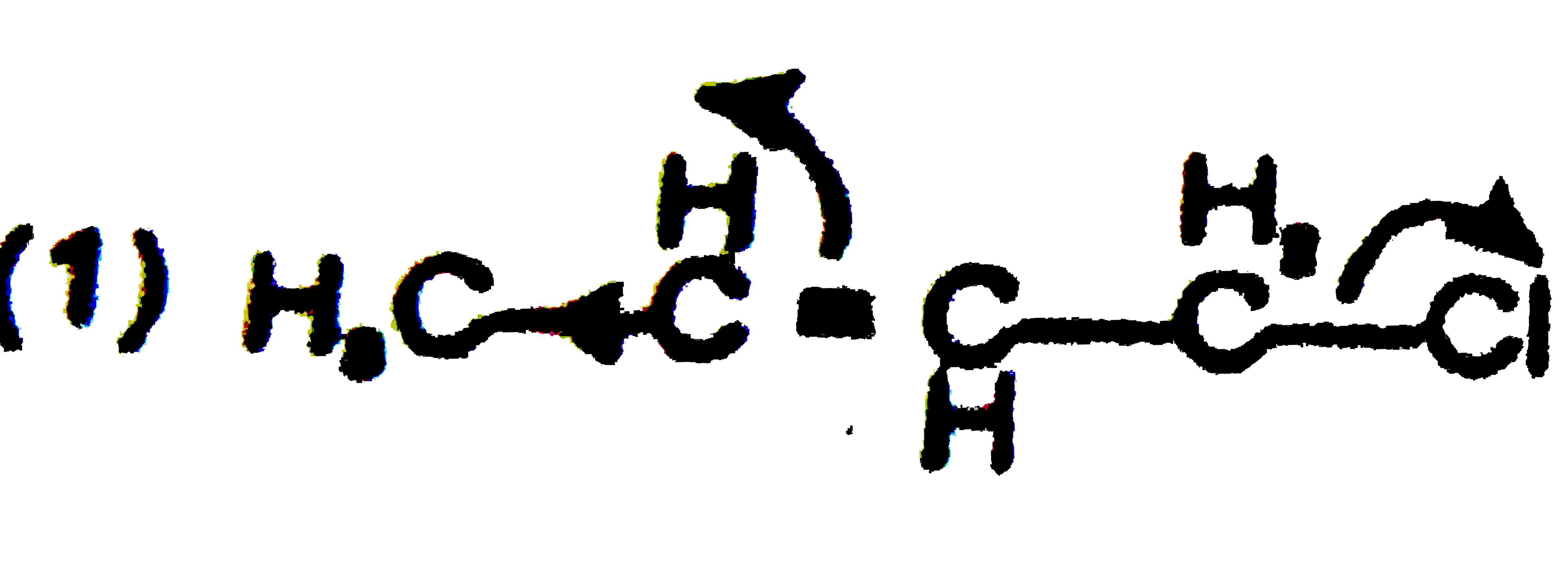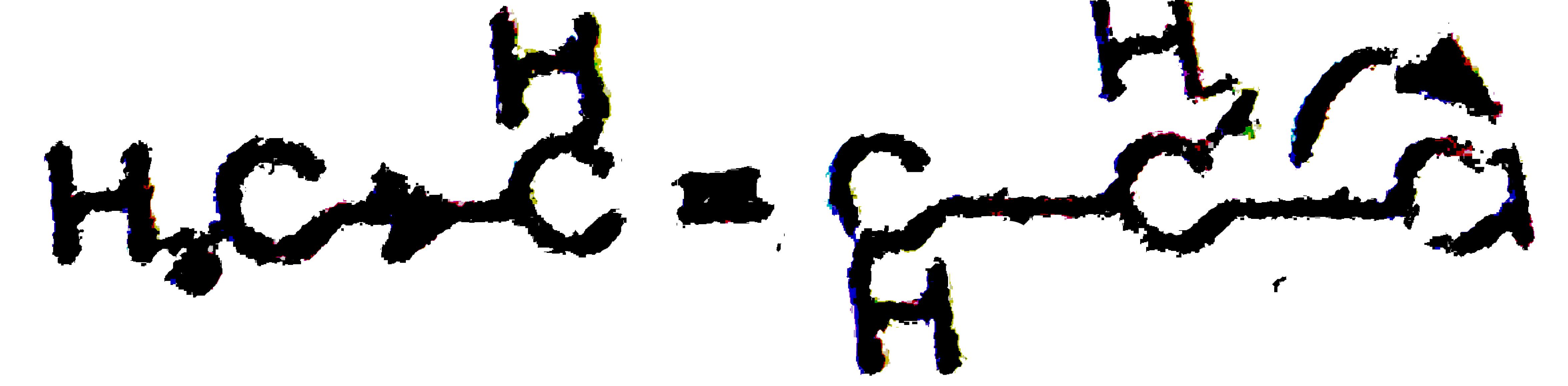A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-HYDROCARBON-ORGANIC CHEMISTRY(Hydrocarbon)
- Which of the following organic compounds has same hybridisation as its...
Text Solution
|
- Identity Z in the sequence of reactions CH(3)CH(2)CH = CH(2) overs...
Text Solution
|
- Which of the following is the most correct electron displacement for a...
Text Solution
|
- Addition of sodium to bromobenzene in dry ether will produce :
Text Solution
|
- The gas with the highest heat of combustion is :
Text Solution
|
- Structural formula for lewisite is
Text Solution
|
- Acetylene react with alkaline KMnO(4) to give
Text Solution
|
- Benzene reacts with CH(3)Cl in the presence of anyhydrous AlCl(3) to f...
Text Solution
|
- Petroleum mainly contains :
Text Solution
|
- Which one of the following is most reactive towards electrophillic rea...
Text Solution
|
- CH3C-=C CH3underset(CaCO3 and BaSO4)overset((H2)/(Pd))to P Identifty t...
Text Solution
|
- The alkyl halides that can be made by free radical halogenation of alk...
Text Solution
|
- The species which acts as electrophile in the bromination of benzene...
Text Solution
|
- Reaction by which benzaldehyde cannot be prepared?
Text Solution
|
- Which of the following carbides on treatment with water give methane ...
Text Solution
|
- Propene, CH(3)-CH = CH(2), can be converted to 1-propanol by oxidation...
Text Solution
|
- What products are formed when the following compound is treated with ...
Text Solution
|
- When HCl gas is passed through propene in the presence of benzoyl pero...
Text Solution
|
- What is the energy in joule of photon of light whose wave length is 1....
Text Solution
|
- The treatment of benzene with isobutene in the presence of sulphuric a...
Text Solution
|