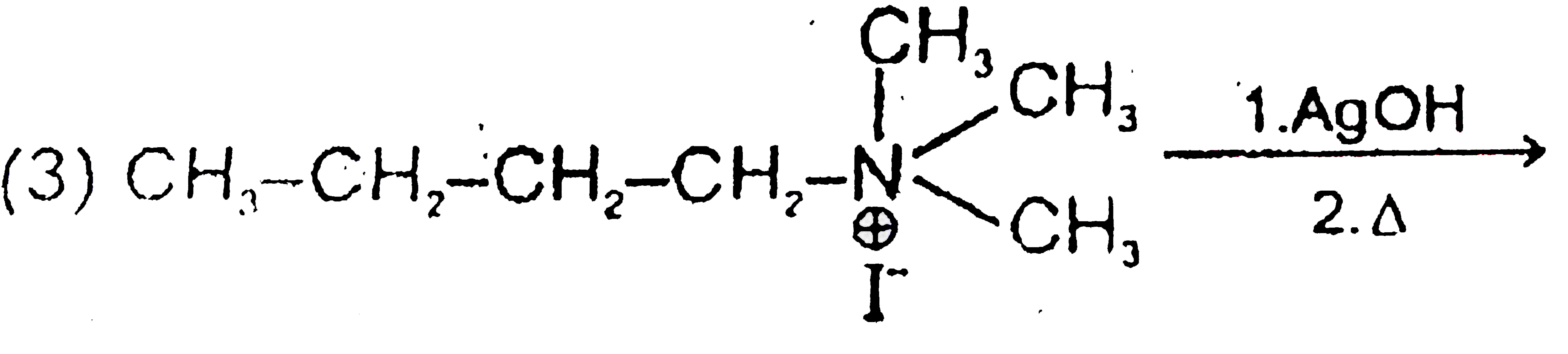
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-HYDROCARBON-ORGANIC CHEMISTRY(Hydrocarbon)
- The threshold wavelength for ejection of electrons from a metal is 230...
Text Solution
|
- The reaction of HBr with gives
Text Solution
|
- In which of the following Hoffmann elimination product is more ?
Text Solution
|
- Which of the following is not aromatic?
Text Solution
|
- Identify Z in the series CH(3)CH(2)CH(2)OHunderset(160-180^(@)C)over...
Text Solution
|
- In the reaction, product 'X' is : CH(3)-C=CH+H(2)Ooverset(H^(+)//Hg^...
Text Solution
|
- For the given reaction how many monochloro products are optically acti...
Text Solution
|
- The threshold wavelength for ejection of electrons from a metal is 350...
Text Solution
|
- Oberve the following reaction sequence
Text Solution
|
- . The product 'W' is:
Text Solution
|
- . The product C is :
Text Solution
|
- The threshold wavelength for ejection of electrons from a metal is 450...
Text Solution
|
- The velocity of electron of H-atom in its ground state is 8.4×10^(5)m/...
Text Solution
|
- The threshold wavelength for ejection of electrons from a metal is 280...
Text Solution
|
- The minimum heat of reaction is required in the formation of :
Text Solution
|
- CH(3)-underset(" "C(2)H(5))underset(|)overset(D)overset(|)(C)-MgBr+CH(...
Text Solution
|
- Using corey - house synthesis we can't prepare ………………….. from ethylbro...
Text Solution
|
- During the electrolysis of sodium ethanoate, the gas liberated at cath...
Text Solution
|
- During Kolbe's electrolytic method, the pH of aqueous solution of sa...
Text Solution
|
- In Kolbe's electrolysis sodium propanoate gives :
Text Solution
|