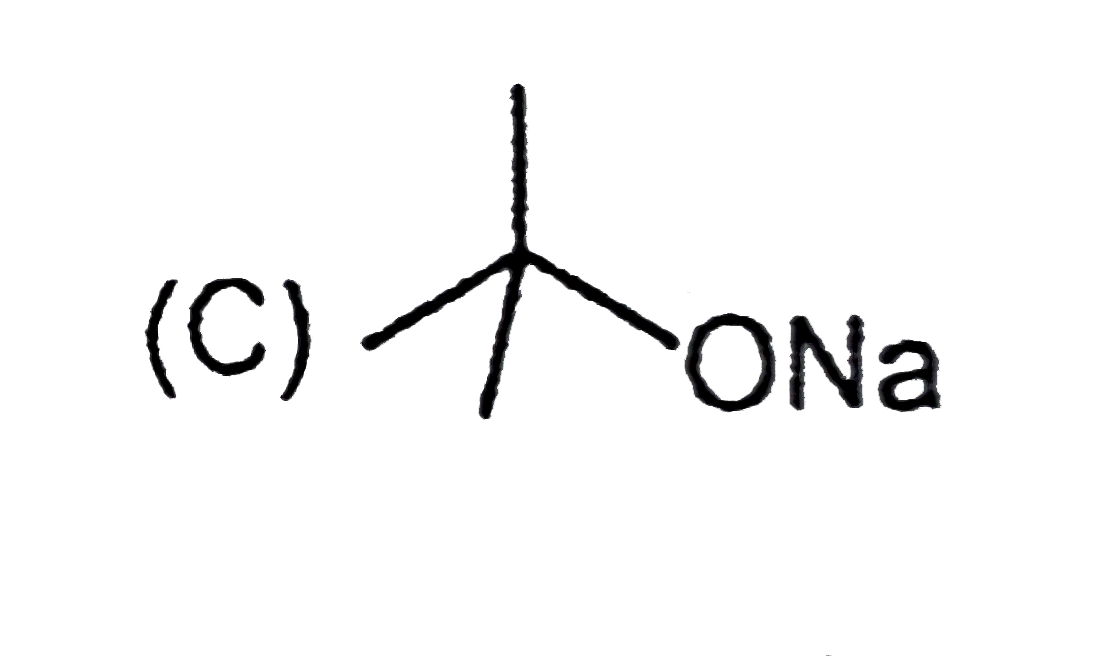
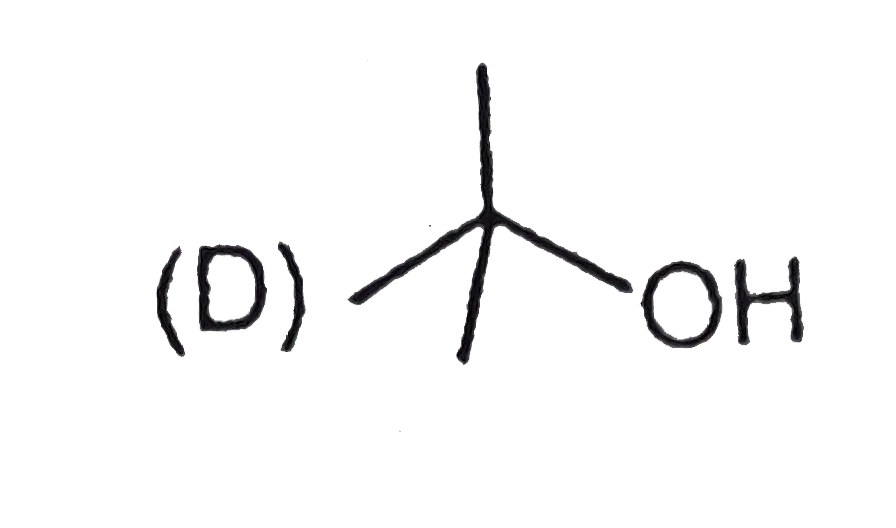
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-TEST SERIES-CHEMISTRY
- Which of the following reactions does not occur during calcination?
Text Solution
|
- Which of the following compounds can show geometrical optical and conf...
Text Solution
|
- Which of the following is the strongest nucleophile?
Text Solution
|
- Which of the following are low spin complexes which follow EAN rule?
Text Solution
|
- Which of the following statement(s) is/are correct?
Text Solution
|
- ^^(m)^(0)H(2)O is equal to
Text Solution
|
- Which of the following is/are oxide ores?
Text Solution
|
- Zn amalgam is prepared by elctrtolysis of aqueous ZnCI(2) using 9 gram...
Text Solution
|
- An aqueous solution of glucose was prepared by dissolving 18 g of gluc...
Text Solution
|
- Which of the following reaction/s give same product?
Text Solution
|
- The correct statements among the following is/are
Text Solution
|
- Which of the following substrates is/are more reactive than ethyl brom...
Text Solution
|
- When mango is placed in dilute aqueous solution of hydrochloric acid, ...
Text Solution
|
- Metallic gold frequently is found in aluminosilicate rocks and it is f...
Text Solution
|
- Which of the following is/are correct for cyanide process of extractio...
Text Solution
|
- What happens to the freezing point of water when a non-volatile solute...
Text Solution
|
- Determine the osmotic pressure of a solution prepared by dissolving 25...
Text Solution
|
- The purity of NaCI solid is checked by measuring boiling point of its ...
Text Solution
|
- Define osmotic pressure, How is osmotic pressure arelated to the conce...
Text Solution
|
- State true or false : The oxidation number of cobalt in K[Co(CO)(4)...
Text Solution
|