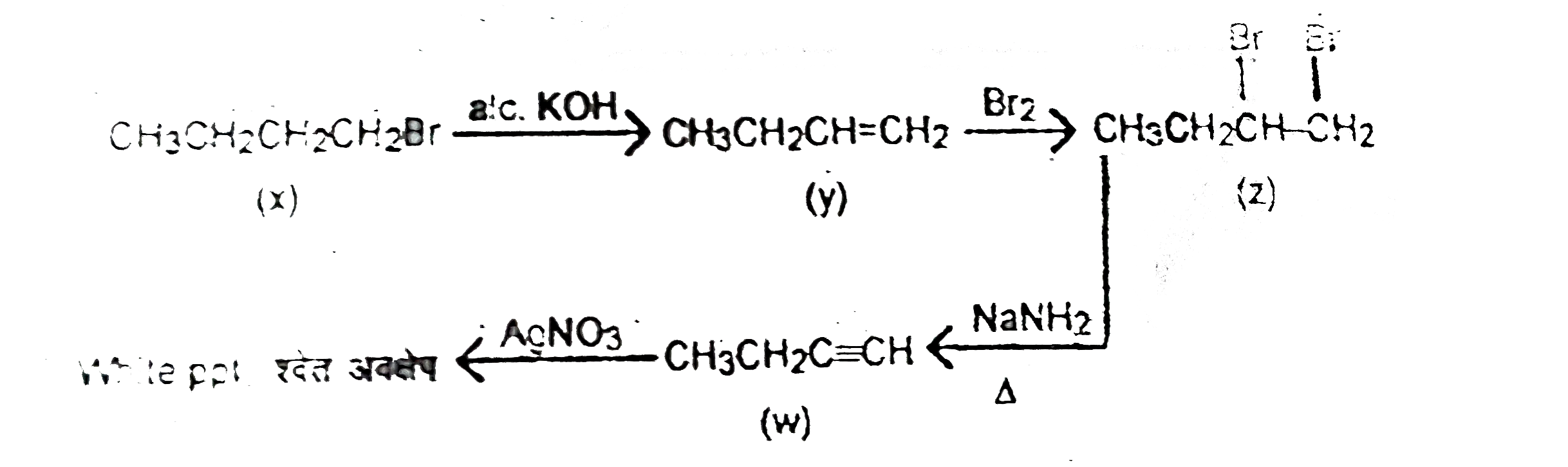
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-TEST SERIES-CHEMISTRY
- Which of the following is/are correct for cyanide process of extractio...
Text Solution
|
- What happens to the freezing point of water when a non-volatile solute...
Text Solution
|
- Determine the osmotic pressure of a solution prepared by dissolving 25...
Text Solution
|
- The purity of NaCI solid is checked by measuring boiling point of its ...
Text Solution
|
- Define osmotic pressure, How is osmotic pressure arelated to the conce...
Text Solution
|
- State true or false : The oxidation number of cobalt in K[Co(CO)(4)...
Text Solution
|
- Choose the incorrect statement about corrosion on the metal surface.
Text Solution
|
- The standard electrode potential for Daniel cell is 1.1V. Calculate th...
Text Solution
|
- Electrode potential for Mg electrode varies according to the equation ...
Text Solution
|
- The number of moles of water which must be electrolyzed to produce 22....
Text Solution
|
- A solution of CHCl3 +CH3 COCH3 shows negative deviation from Rao...
Text Solution
|
- Select in incorrect match
Text Solution
|
- Select the incorrect statement.
Text Solution
|
- Silicones are water repelling in nature due to.
Text Solution
|
- The colour developed when Na(2)S is added to Na(2)[Fe(CN)(5)NO] is,
Text Solution
|
- An aqueous solution of glucose boils at 100.04^(@)C.The molal elevatio...
Text Solution
|
- Which of the following interfaces cannot be obtained ?
Text Solution
|
- Which of the following statement is true?
Text Solution
|
- CH(3)-CH(2)-OH In the given reaction A & B are respectively:
Text Solution
|
- Which destroy antigens?
Text Solution
|