A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-TEST SERIES-CHEMISTRY
- Choose the incorrect statement about corrosion on the metal surface.
Text Solution
|
- The standard electrode potential for Daniel cell is 1.1V. Calculate th...
Text Solution
|
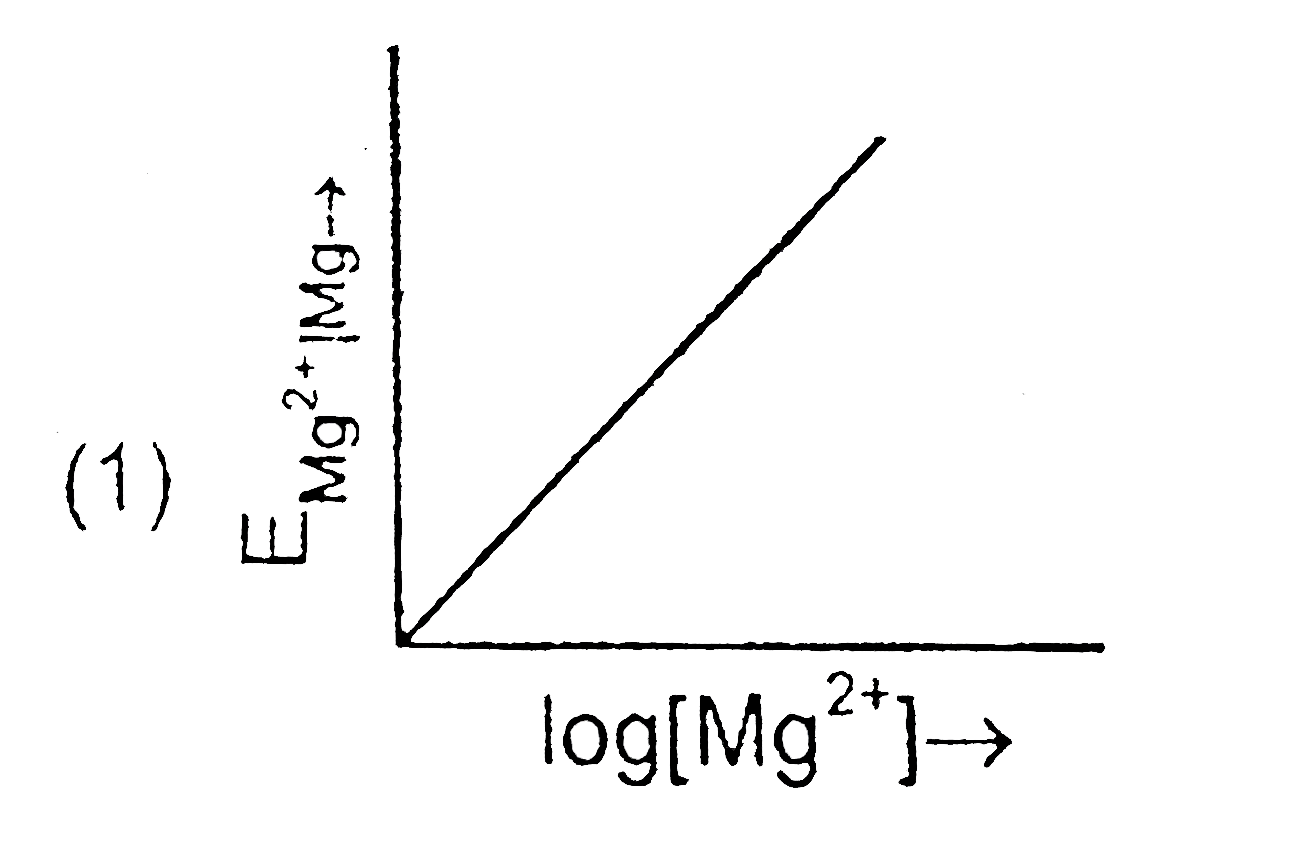
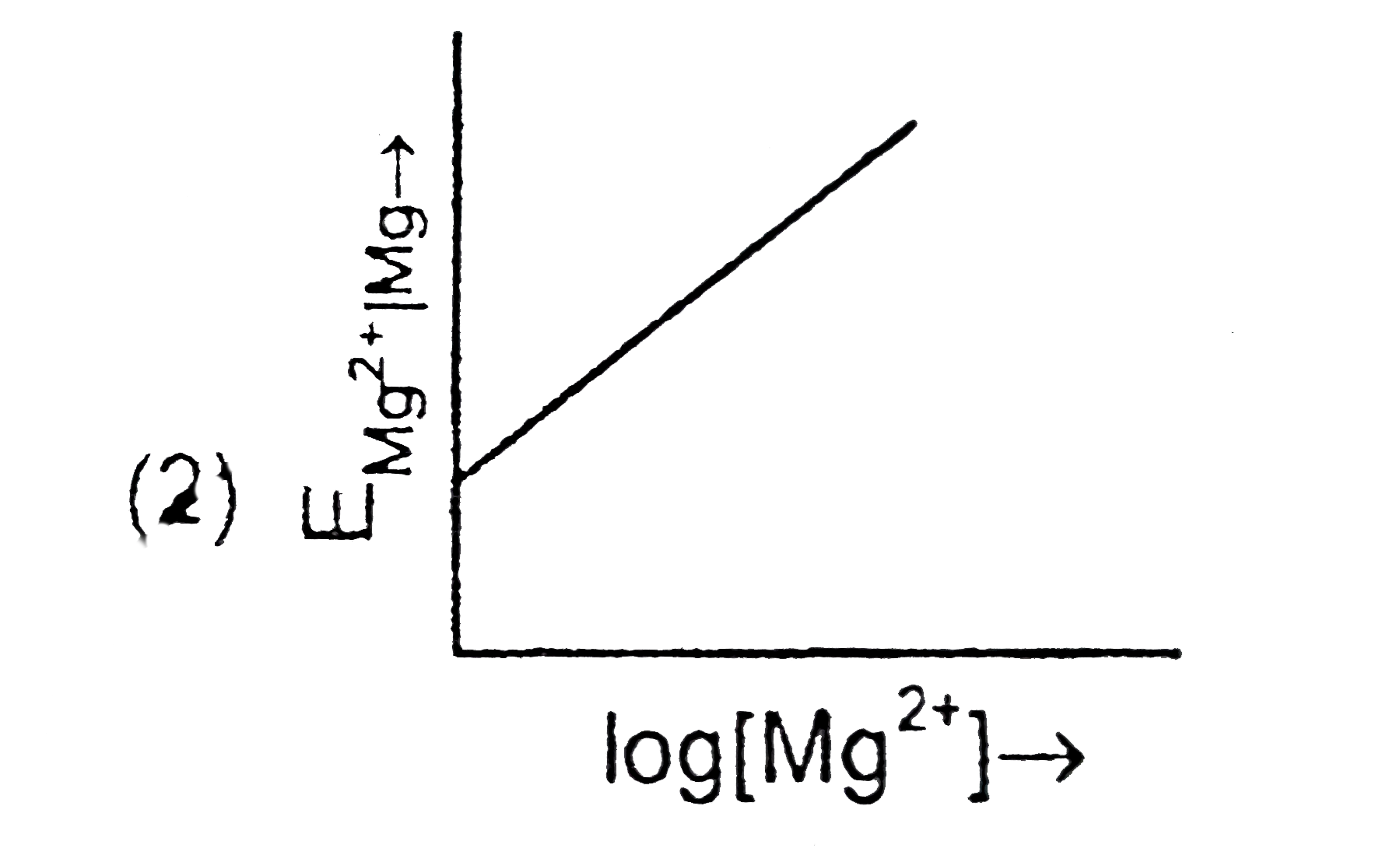
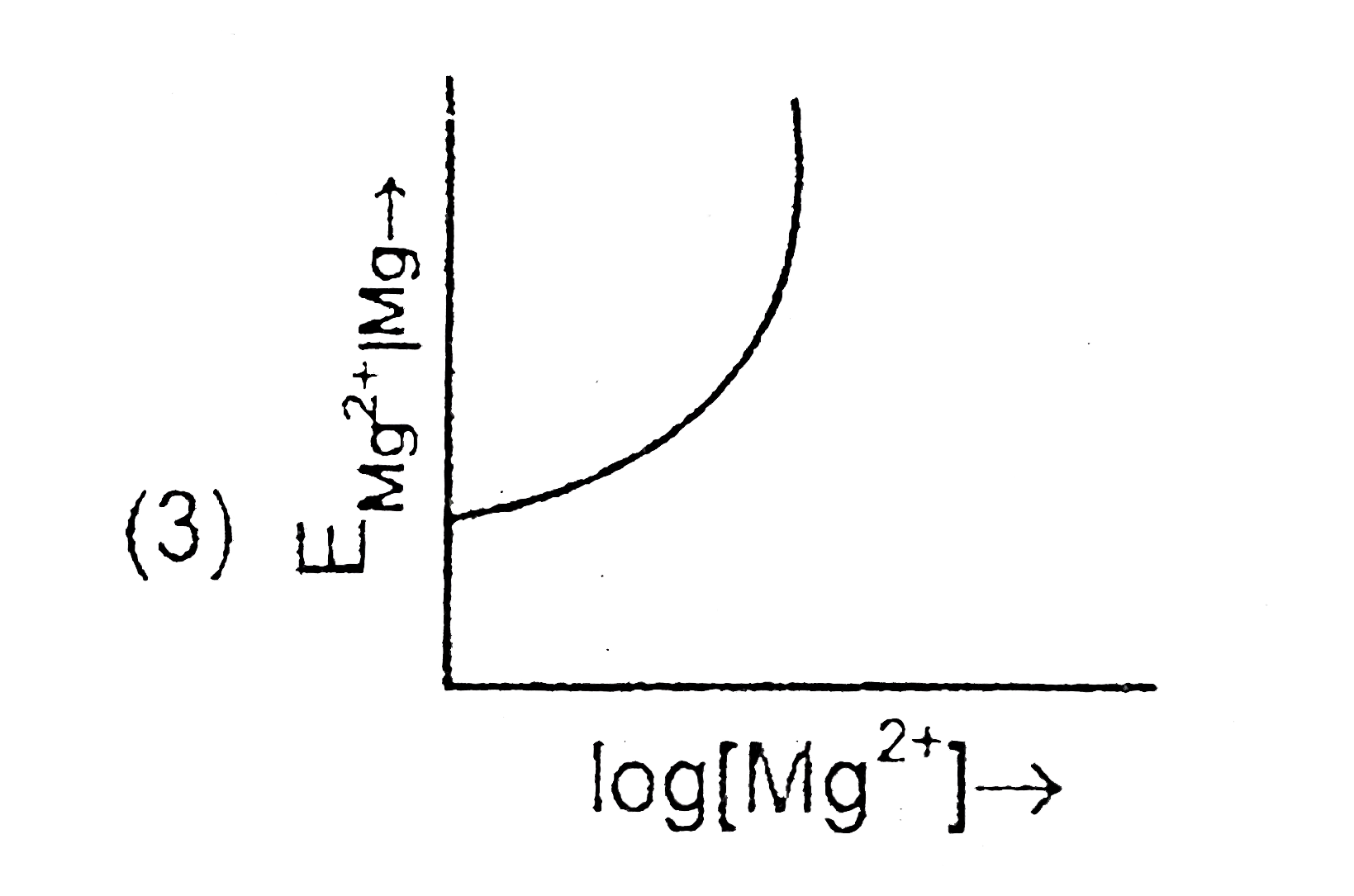
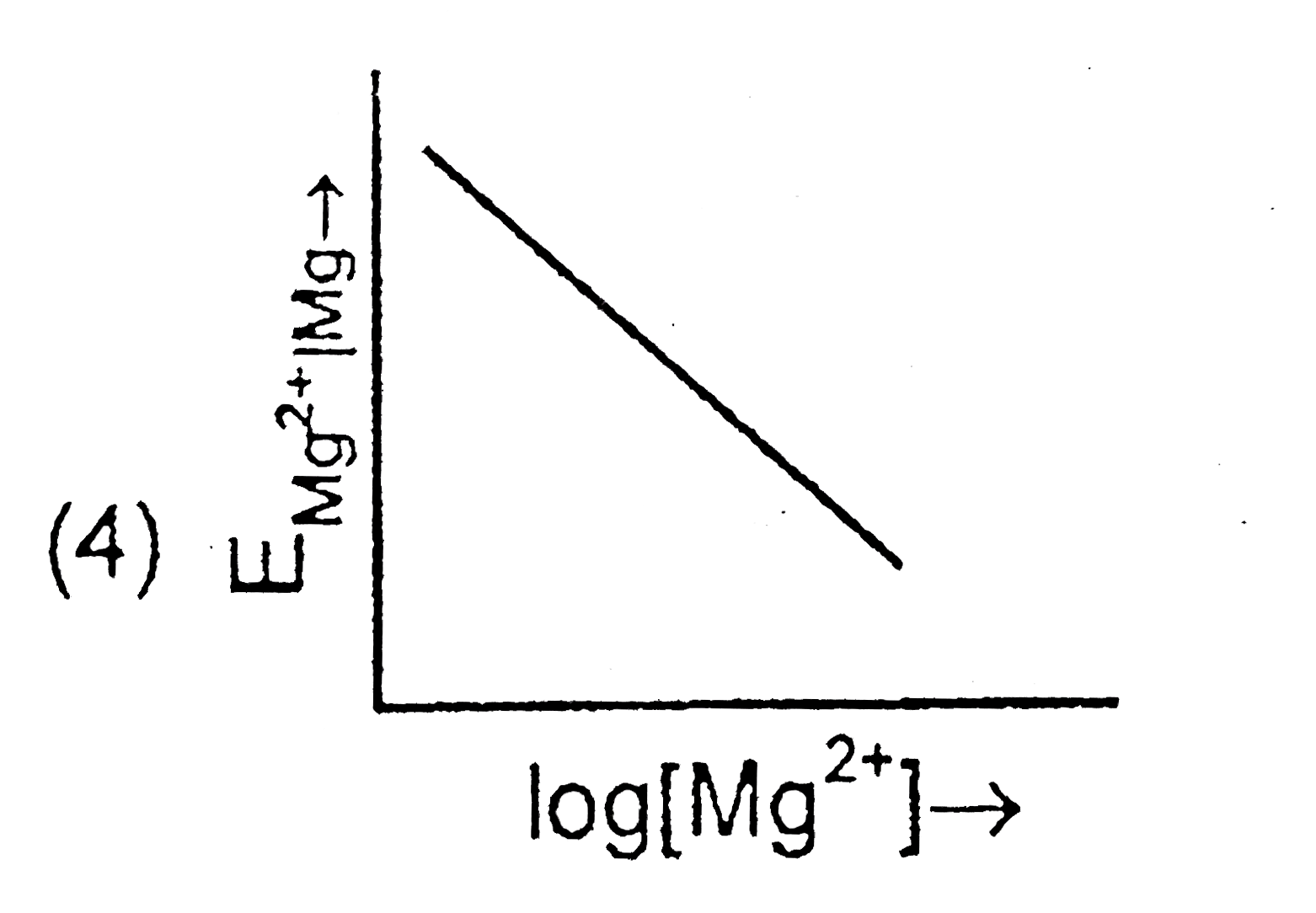
- Electrode potential for Mg electrode varies according to the equation ...
Text Solution
|
- The number of moles of water which must be electrolyzed to produce 22....
Text Solution
|
- A solution of CHCl3 +CH3 COCH3 shows negative deviation from Rao...
Text Solution
|
- Select in incorrect match
Text Solution
|
- Select the incorrect statement.
Text Solution
|
- Silicones are water repelling in nature due to.
Text Solution
|
- The colour developed when Na(2)S is added to Na(2)[Fe(CN)(5)NO] is,
Text Solution
|
- An aqueous solution of glucose boils at 100.04^(@)C.The molal elevatio...
Text Solution
|
- Which of the following interfaces cannot be obtained ?
Text Solution
|
- Which of the following statement is true?
Text Solution
|
- CH(3)-CH(2)-OH In the given reaction A & B are respectively:
Text Solution
|
- Which destroy antigens?
Text Solution
|
- Rate of S(N)1 & S(N)2 reactions for the isomers of C(4)H(9)Br is i....
Text Solution
|
- Chloroquine is:
Text Solution
|
- The osmotic pressure of a dilute solution is given by
Text Solution
|
- 3 gms of urea is dissolved in 45 gms of H2O. The relative lowering in ...
Text Solution
|
- An aqueous solution of glucose boils at 100.05^(@)C.The molal elevatio...
Text Solution
|
- CH(3)-underset(CH(3)) underset(|) (C)= CH(2) underset("Peroxide") over...
Text Solution
|