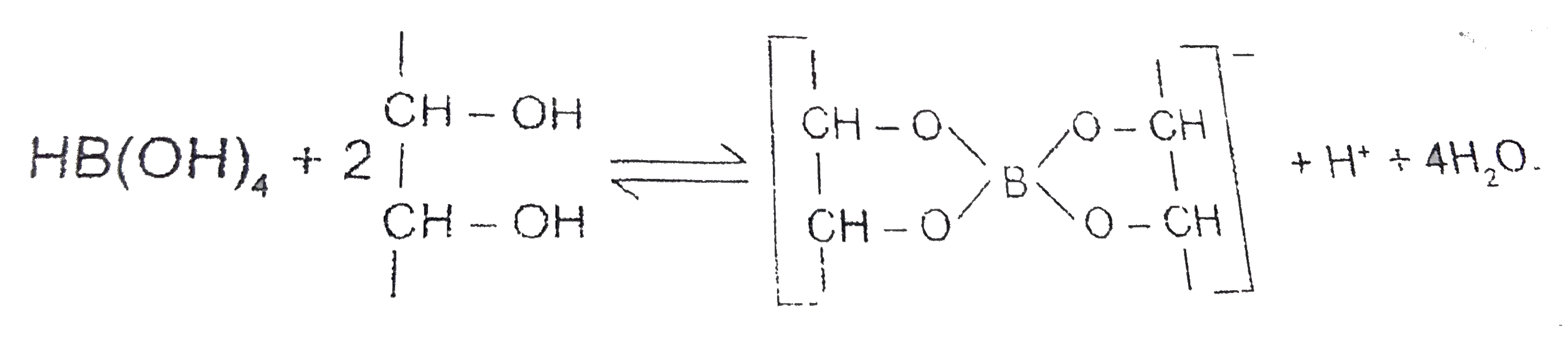
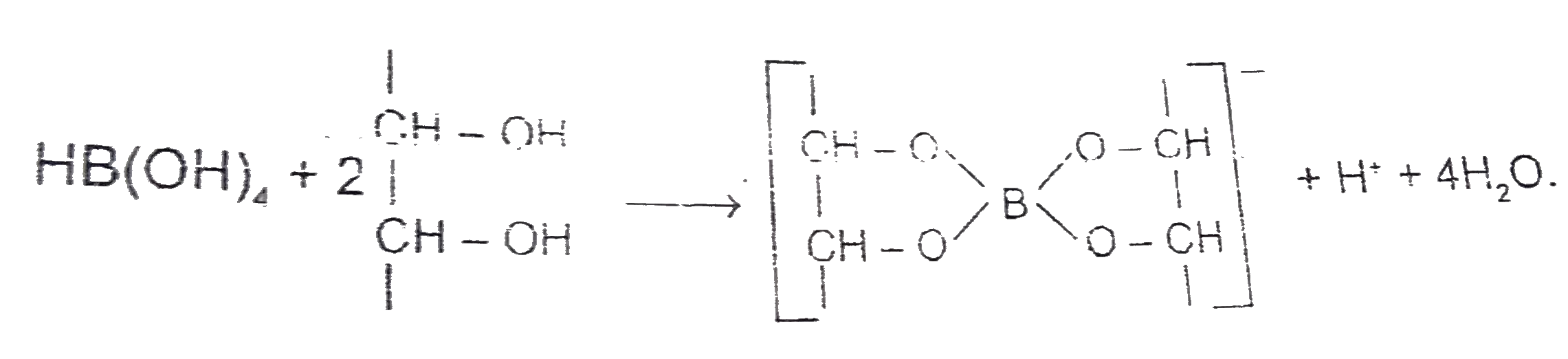
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P-BLOCK ELEMENT (BORON AND CARBON FAMILY)
RESONANCE ENGLISH|Exercise Exercise-3|17 VideosP-BLOCK ELEMENT (BORON AND CARBON FAMILY)
RESONANCE ENGLISH|Exercise Exercise-3 Part-II : JEE (MAIN) / AIEE PROBLEMS (PREVIOUS YEARS)|12 VideosP-BLOCK ELEMENT (BORON AND CARBON FAMILY)
RESONANCE ENGLISH|Exercise Exercise-2 Part-III : ONE OR MORE THAN ONE OPTIONS CORRECT TYPE|26 VideosP BLOCK ELEMENTS
RESONANCE ENGLISH|Exercise PART -II|23 VideosP-BLOCK ELEMENTS (HALOGEN & NOBLE GASES)
RESONANCE ENGLISH|Exercise APSP PART-3|22 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-P-BLOCK ELEMENT (BORON AND CARBON FAMILY)-Exercise-2 Part-IV : COMPREHENSIONS
- Compound (A) on reaction with iodine in the solvent diglyme gives a hy...
Text Solution
|
- Compound (A) on reaction with iodine in the solvent diglyme gives a hy...
Text Solution
|
- Compound (A) on reaction with iodine in the solvent diglyme gives a hy...
Text Solution
|
- All the boron trihalides except BI(3) may be prepared by direction be...
Text Solution
|
- All the boron trihalides except BI(3) may be prepared by direction be...
Text Solution
|
- Which is correct about the hydrolysis of BX(3)?
Text Solution
|
- All the boron trihalides except BI(3) may be prepared by direction be...
Text Solution
|
- The small size and high charge of Al^(3+) ion gives it a high charge d...
Text Solution
|
- Which one of the following statements is correct ?
Text Solution
|
- The small size and high charge of Al^(3+) ion gives it a high charge d...
Text Solution
|
- The small size and high charge of Al^(3+) ion gives it a high charge d...
Text Solution
|