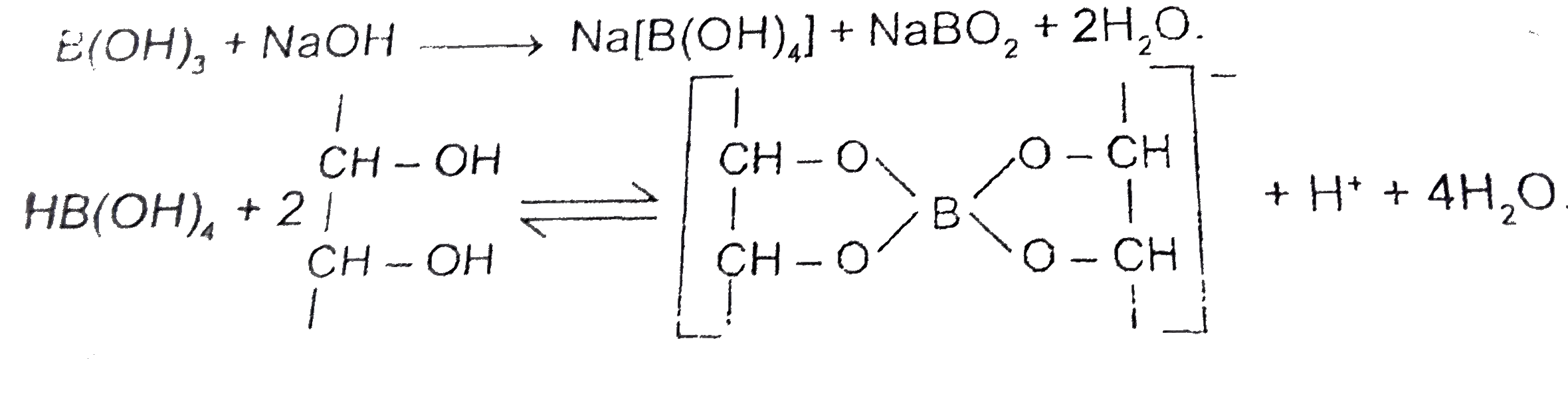A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P-BLOCK ELEMENT (BORON AND CARBON FAMILY)
RESONANCE ENGLISH|Exercise Exercise-3 Part-II : JEE (MAIN) / AIEE PROBLEMS (PREVIOUS YEARS)|12 VideosP-BLOCK ELEMENT (BORON AND CARBON FAMILY)
RESONANCE ENGLISH|Exercise Advanced Level Problems Part-I : PRACTICE TEST-1 (IIT-JEE (MAIN Pattern))|28 VideosP-BLOCK ELEMENT (BORON AND CARBON FAMILY)
RESONANCE ENGLISH|Exercise Exercise-2 Part-IV : COMPREHENSIONS|11 VideosP BLOCK ELEMENTS
RESONANCE ENGLISH|Exercise PART -II|23 VideosP-BLOCK ELEMENTS (HALOGEN & NOBLE GASES)
RESONANCE ENGLISH|Exercise APSP PART-3|22 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-P-BLOCK ELEMENT (BORON AND CARBON FAMILY)-Exercise-3
- Write the chemical reactions associated with the 'borax' best 'test' o...
Text Solution
|
- Compound (X) on reduction with LiAlH(4) gives a hydride (Y) containing...
Text Solution
|
- Write the balanced equations for the reactions of the following compou...
Text Solution
|
- Draw the structure of B(2)H(6) and give its reaction with HCl.
Text Solution
|
- H(3)BO(3) is :
Text Solution
|
- B(OH)(3)+NaOH to Na[B(OH)(4)](aq). Then addition of which of the fo...
Text Solution
|
- Statement-I : In water, orthoboric acid behaves as a weak monobasic ac...
Text Solution
|
- The coordination number of Al in the crystalline state of AlCl(3) is .
Text Solution
|
- The correct statement(s) for orthoboric acid is/are :
Text Solution
|
- Starting from SiCl4 prepare the following in steps not exceeding the n...
Text Solution
|
- Me(2)SiCl(2) on hydrolysis will produce :
Text Solution
|
- In SiO(4)^(4-) the tetrahedral molecule two oxygen atoms are shared in
Text Solution
|
- Statement I: Pb^(4+) compounds are stronger oxidizing agents than Sn^(...
Text Solution
|
- In the reaction : 2X+B(2)H(6) rarr [BH(2)(X(2))]^(oplus) [BH(4)]^(Ө)...
Text Solution
|
- The value of n in the molecular formula Be(n)Al(2) Si( 6)O(18) is …..
Text Solution
|
- Three moles of B(2)H(6) are completely reacted with methanol. The nu...
Text Solution
|
- Under hydrolytic conditions, the compounds used for preparation of lin...
Text Solution
|