A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL EQUILIBRIUM
RESONANCE ENGLISH|Exercise Exercise-2 (Part-2)|22 VideosCHEMICAL EQUILIBRIUM
RESONANCE ENGLISH|Exercise Exercise-2 (Part-3)|33 VideosCHEMICAL EQUILIBRIUM
RESONANCE ENGLISH|Exercise Exercise-1 (Part-2)|87 VideosCHEMICAL BONDING
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Fundamental Concept )|6 VideosD & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS
RESONANCE ENGLISH|Exercise Match the column|1 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-CHEMICAL EQUILIBRIUM-Exercise-2 (Part-1)
- If for 2A(2)B(g)hArr2A(2)(g)+B(2)(g),K(P)="TOTAL PRESSURE" ("at equili...
Text Solution
|
- Ammonia gas at 15 atm is introduced in a rigid vessel at 300 K. At equ...
Text Solution
|
- Attainment of the equilibrium A(g)hArr2C(g)+B(g)gave the following gra...
Text Solution
|
- A 10 L "container at" 300K "contains" CO(2) "gas at pressure of" 0.2 "...
Text Solution
|
- Two solid A "and" B are present in two different container having same...
Text Solution
|
- To the system, LaCl(3)(s)+H(2)O(g) hArr LaClO(s)+2HCL(g)-"Heat" alre...
Text Solution
|
- Some quantity of water is contained in a container as shown in figure....
Text Solution
|
- The equilibrium constant for, 2H(2)S(g)hArr2H(2)(g)+S(2)(g) "is" 0.011...
Text Solution
|
- For reaction, assuming large volume of water. H(2)O(l)hArrH(2)O(g) ,...
Text Solution
|
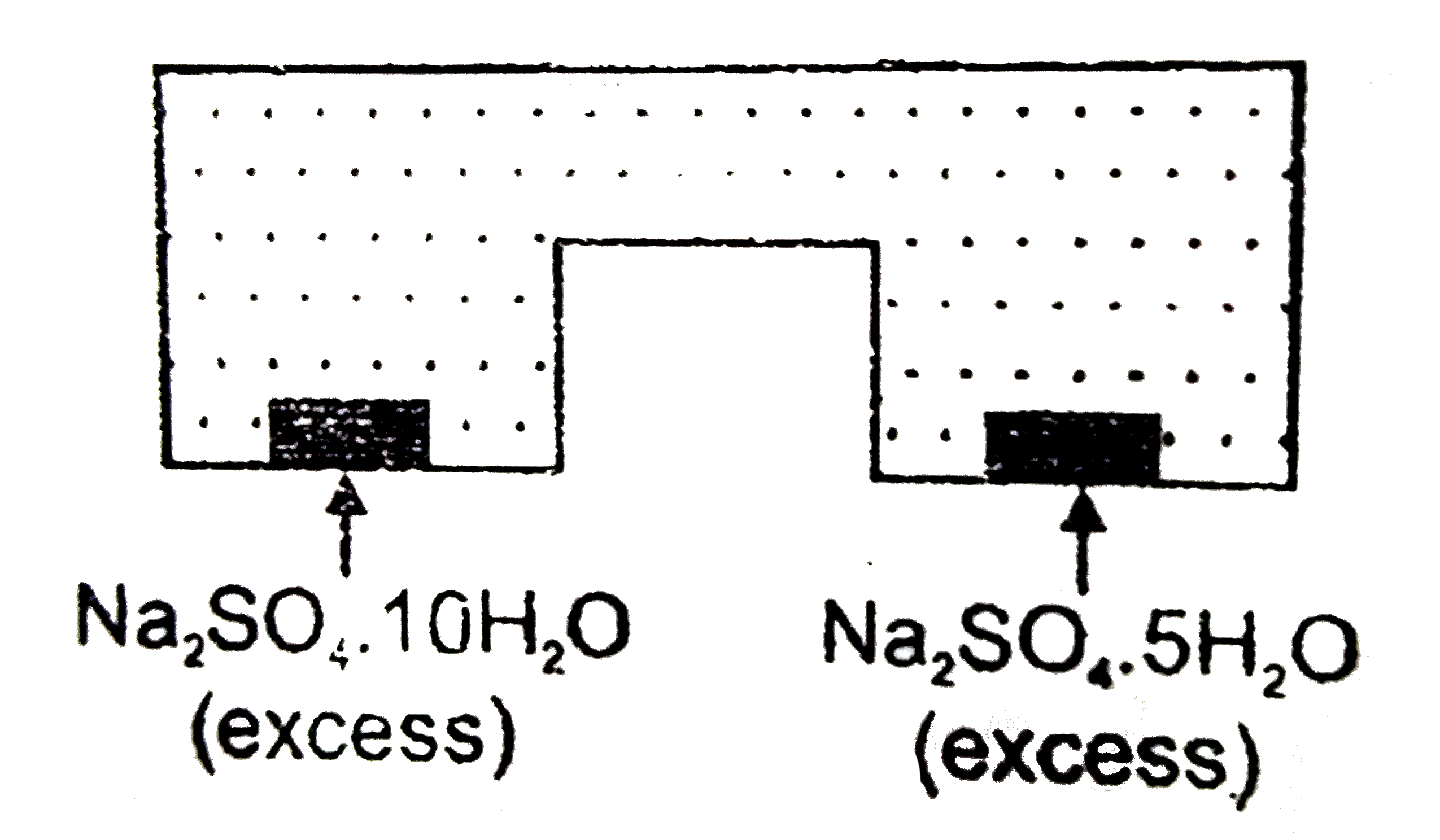
- Na(2)SO(4).10H(2)O(s)hArrNa(2)SO(4).5H(2)O(g) K(P)=2.43xx10^(-8) atm^(...
Text Solution
|
- For equilibrium ZnSO(4).7H(2)O(s)hArrZnSO(4).2H(2)O(s)+5H(2)O(g) K(P...
Text Solution
|
- In the Haber process for the industrial manufacturing of ammonia invol...
Text Solution
|
- Addition of water to which of the following equilibria causes it to sh...
Text Solution
|
- Consider the reactions (i) PCl(5)(g) hArr PCl(3)(g)+Cl(2)(g) (ii) ...
Text Solution
|
- Solid A "and" B are taken in a closed container at a certain temperatu...
Text Solution
|
- For a system at equilibrium some changes are made which is reported by...
Text Solution
|
- In one experiment, certain amount of NH(4)I(s) was heated rapidly in a...
Text Solution
|
- A(s)hArrB(g)+C(g) K(P)=40atm^(2) X(s)hArrB(g)+E(g) Above equilibri...
Text Solution
|
- X(s)hArrY(g)+2Z(g) A(s)hArrY(g)+B(g) Consider both these equilibri...
Text Solution
|
- 1-butyne and 1, 3-butadiene are :
Text Solution
|